Single arm prospective multicenter case series on the use of burst stimulation to improve pain and motor symptoms in Parkinson's disease
- PMID: 33005705
- PMCID: PMC7520952
- DOI: 10.1186/s42234-020-00055-3
Single arm prospective multicenter case series on the use of burst stimulation to improve pain and motor symptoms in Parkinson's disease
Abstract
Background: In this study we analyze new clinical data in the use of spinal cord stimulation (SCS) for the treatment of pain and motor symptoms in patients with Parkinson's Disease (PD), as both a singular bioelectric therapy and as a salvage therapy after deep brain stimulation (DBS).
Methods: Fifteen patients were recruited and had percutaneous electrodes implanted at the level of the thoracic or cervical spine. Participants were set to one of three stimulation modes: continuous tonic stimulation, continuous Burst stimulation (40 Hz, 500 Hz, 1000 μs), or cycle mode (on time of 10-15 s, off time of 15-30 s) with Burst (40 Hz, 500 Hz, 1000 μs). Patients completed the Visual Analogue Scale (VAS), Unified Parkinson's Disease Rating Scale, Self-Rating Depression Scale, Hamilton Depression Rating Scale, Profile of Mood State, 10-meter walking test, and the Timed Up and Go (TUG).
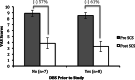
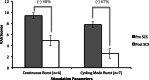
Results: All patients experienced significant improvement in VAS scores with a mean reduction of 59% across all patients. Patients who chose the cycling burst stimulation parameter had an average 67% reduction in VAS scores, as compared to the continuous burst parameter group, which had an average 48% reduction in VAS scores. Seventy-three percent of patients experienced improvement in the 10-meter walk, with an average improvement of 12%. Sixty-four percent of patients experienced clinically relevant improvements in the TUG, with an average improvement of 21%.
Conclusions: This study points to the potential utility of SCS to address both pain and certain aspects of motor symptoms in PD patients who have and have not received DBS therapy.
Keywords: Burst simulation; Deep brain stimulation; Dorsal column stimulation; Parkinson disease; Spinal cord stimulation.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsDr. Chakravarthy is a consultant to Abbott, Bioness, Medtronic, Nalu Medical, Saluda Medical. He has stock options in Nalu Medical. He is also founder of Newrom Biomedical. Dr. Iwamuro’s workplace, The Department of Research and Therapeutics for Movement Disorders at the Juntendo University Graduate School of Medicine, is an endowment department supported with an unrestricted grant from Medtronic, Boston Scientific, Kyowa Kirin, Boehringer Ingelheim, AbbVie and FP Pharmaceutical. Dr. Ayano Matsui has received honoraria for lectures and writing about SCS from Abbott Medical Japan. There are no other reported conflicts of interest for this body of work from the other authors.
Figures
References
LinkOut - more resources
Full Text Sources
Medical