Plasma amyloid, tau, and neurodegeneration biomarker profiles predict Alzheimer's disease pathology and clinical progression in older adults without dementia
- PMID: 33005724
- PMCID: PMC7513626
- DOI: 10.1002/dad2.12104
Plasma amyloid, tau, and neurodegeneration biomarker profiles predict Alzheimer's disease pathology and clinical progression in older adults without dementia
Abstract
Introduction: Plasma markers have been reported to be associated with brain amyloid burden, tau pathology, or neurodegeneration. We aimed to evaluate whether plasma biomarker profiles could predict Alzheimer's disease (AD) pathology and clinical progression in older adults without dementia.
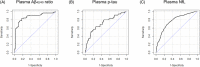
Methods: Cross-sectional and longitudinal data of participants enrolled in this study were from the Alzheimer's Disease Neuroimaging Initiative (ADNI). Plasma amyloid beta (Aβ)1-42/Aβ1-40 ratio was selected as the marker for amyloid pathology, p-tau181 for tau pathology, and neurofilament light for neurodegeneration. Cut-offs for these plasma markers were calculated with well-established positron emission tomography and structural imaging biomarkers as reference. Older adults without dementia were categorized into eight groups at baseline by plasma amyloid/tau/neurodegeneration (A/T/N) cut-offs. Clinical progression was analyzed using linear mixed-effects models and Cox proportional hazard models.
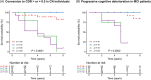
Results: A total of 183 participants (97 cognitively normal [CN] subjects and 86 patients with mild cognitive impairment [MCI]; mean age 72.6 years, and 48.1% men) were included. Participants with A+ had significantly higher proportions of apolipoprotein E (APOE) gene ɛ4 carriers than those with A-. Brain atrophy was observed in all groups of CN, whereas cognition decline was obvious in the A+T+N+ group. Compared to A-T-N-, MCI patients with A+T+N+ had faster cognition worsening and faster brain atrophy. In the whole cohort, A+T+N+ and A+T+N- participants were at higher risk of clinical progression.
Discussion: Plasma A/T/N biomarker profiles may predict AD pathology and clinical progression, indicating a potential role for plasma biomarkers in clinical trials. More research is warranted to develop a robust plasma AD framework.
Keywords: Alzheimer's disease; amyloid beta; mild cognitive impairment; neurofilament light; plasma; p‐tau181.
© 2020 The Authors. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals, LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


References
-
- Scheltens P, Blennow K, Breteler MM, et al. Alzheimer's disease. Lancet. 2016;388:505‐517. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
