Secretion of pro-oncogenic AGR2 protein in cancer
- PMID: 33005802
- PMCID: PMC7519367
- DOI: 10.1016/j.heliyon.2020.e05000
Secretion of pro-oncogenic AGR2 protein in cancer
Abstract
Anterior gradient-2 (AGR2) protein mediates the formation, breakage and isomerization of disulphide bonds during protein maturation in the endoplasmic reticulum (ER) and contributes to the homoeostasis of the secretory pathway. AGR2 promotes tumour development and metastasis and its elevated expression is almost completely restricted to malignant tumours. Interestingly, this supposedly ER-resident protein can be localised to other compartments of cancer cells and can also be secreted into the extracellular milieu. There are emerging evidences that describe the gain-of-function activities of the extracellular AGR2, particularly in cancer development. Here, we reviewed studies detailing the expression, pathological and physiological roles associated with AGR2 and compared the duality of localization, intracellular and extracellular, with special emphasis on the later. We also discussed the possible mechanisms of AGR2 secretion as well as deliberating the functional impacts of AGR2 in cancer settings. Last, we deliberate the current therapeutic strategies and posit the potential use AGR2, as a prognosis and diagnosis marker in cancer.
Keywords: Biochemistry; Cancer; Cancer research; Cell biology; Enzymology; Molecular biology; Oncology; PDI; Protein folding; Protein secretion; Secretory pathway; UPR.
© 2020 The Author(s).
Figures

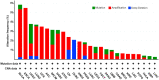
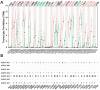
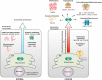
References
-
- Dancourt J., Barlowe C. Protein sorting receptors in the early secretory pathway. Annu. Rev. Biochem. 2010;79:777–802. - PubMed
-
- Hipp M.S., Kasturi P., Hartl F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 2019;20:421–435. - PubMed
-
- Moenner M., Pluquet O., Bouchecareilh M., Chevet E. Integrated endoplasmic reticulum stress responses in cancer. Canc. Res. 2007;67:10631–10634. - PubMed
-
- Dejeans N., Manié S., Hetz C., Bard F., Hupp T., Agostinis P., Samali A., Chevet E. Addicted to secrete – novel concepts and targets in cancer therapy. Trends Mol. Med. 2014;20:242–250. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

