Heat shock protein 70 positively regulates transforming growth factor-α-induced hepatocellular carcinoma cell migration via the AKT signaling pathway
- PMID: 33005803
- PMCID: PMC7519371
- DOI: 10.1016/j.heliyon.2020.e05002
Heat shock protein 70 positively regulates transforming growth factor-α-induced hepatocellular carcinoma cell migration via the AKT signaling pathway
Abstract
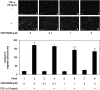
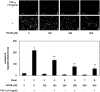
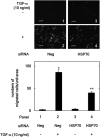
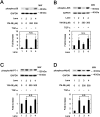
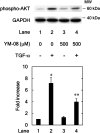
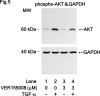
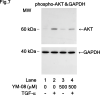
Heat shock proteins (HSPs) are induced in response to extracellular stress and manage the quality of proteins as molecular chaperones. HSP70, a highly conserved HSP, has been reported to correlate with the proliferation and migration of human cancer cells, such as oral, prostate, lung and liver cancer. Regarding hepatocellular carcinoma (HCC), the HSP70 levels in the tumor tissues from patients are significantly higher than those in the normal liver tissues. HSP70 reportedly upregulates the migration and invasion of HCC. The AKT, p38 mitogen-activated protein kinase (MAPK), c-jun N-terminal kinase (JNK) and Rho-kinase signaling pathways regulate the transforming growth factor (TGF)-α-induced migration of human HCC-derived HuH7 cells. However, the exact mechanism underlying the role of HSP70 in growth factor-induced HCC migration remains unclear. Therefore, in the present study, the mechanism underlying the involvement of HSP70 in TGF-α-induced HCC cell migration was investigated. Treatment with the HSP70 inhibitors VER155008 and YM-08 and the downregulation of HSP70 protein were confirmed to significantly suppress the TGF-α-induced cell migration of HuH7 cells. Both VER155008 and YM-08 reduced the TGF-α-induced phosphorylation of AKT without affecting the phosphorylation of p38 MAPK, JNK or Rho-kinase. These results strongly suggest that HSP70 positively regulates the TGF-α-induced migration of HCC cells via the AKT signaling pathway.
Keywords: AKT; Biochemistry; Cancer research; Cell biology; Cell migration; HCC; HSP70; Laboratory medicine; Oncology; TGF-α.
© 2020 The Author(s).
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

