Creating a safe workplace by universal testing of SARS-CoV-2 infection in asymptomatic patients and healthcare workers in the electrophysiology units: a multi-center experience
- PMID: 33006086
- PMCID: PMC7529320
- DOI: 10.1007/s10840-020-00886-9
Creating a safe workplace by universal testing of SARS-CoV-2 infection in asymptomatic patients and healthcare workers in the electrophysiology units: a multi-center experience
Abstract
Background: As the coronavirus cases continue to surge, the urgent need for universal testing to identify positive cases for effective containment of this highly contagious pandemic has become the center of attention worldwide. However, in spite of extensive discussions, very few places have even attempted to implement it. We evaluated the efficacy of widespread testing in creating a safe workplace in our electrophysiology (EP) community. Furthermore, we assessed the new infection rate in patients undergoing EP procedure, to see if identification and exclusion of positive cases facilitated establishment of a risk-free operating environment.
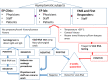
Methods: Viral-RNA and serology tests were conducted in 1670 asymptomatic subjects including patients and their caregivers and staff in our EP units along with the Emergency Medical Service (EMS) staff.
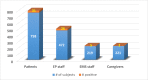
Results: Of 1670, 758 (45.4%) were patients and the remaining 912 were caregivers, EMS staff, and staff from EP clinic and lab. Viral-RNA test revealed 64 (3.8%) positives in the population. A significant increase in positivity rate was observed from April to June 2020 (p = 0.02). Procedures of positive cases (n = 31) were postponed until they tested negative at retesting. Staff testing positive (n = 33) were retested before going back to work after 2 weeks. Because of suspected exposure, 67 staff were retested and source was traced. No new infections were reported in patients during or within 2 weeks after the hospital-stay.
Conclusions: Universal testing to identify positive cases was helpful in creating and maintaining a safe working environment without exposing patients and staff to new infections in the EP units.
Trial registration: Trial Registration Number: clinicaltrials.gov : NCT04352764.
Keywords: Asymptomatic; COVID-19; Electrophysiology; Serology; Universal testing; Viral-RNA.
© 2020. Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Natale A: consulting fees/honoraria: Abbott Medical, Boston Scientific, Medtronic, Biotronik, Biosense Webster, Baylis
Di Biase L: consultant/Advisory Board; Biosense Webster, Hansen Medical
J.D. Burkhardt: Biosense Webster, Boehringer Ingelheim, Stereotaxis
R. Horton: Biosense Webster, St. Jude Medical, Toray, Boston Scientific & Hansen.
D.Lakkireddy: consultant/speaker—Abbott, Medtronic, Boston Scientific, Biotronik, Biosense Webster, North East Scientific, Alta-Thera, Janssen
R. Gopinathannair: Abbott/ Biotronik
Figures

References
-
- Heinzerling A, Stuckey MJ, Scheuer T, Xu K, Perkins KM, Resseger H, Magill S, Verani JR, Jain S, Acosta M, Epson E. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient - Solano County, California, February 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):472–476. doi: 10.15585/mmwr.mm6915e5. - DOI - PMC - PubMed
-
- Gudbjartsson DF, Helgason A, Jonsson H, Magnusson OT, Melsted P, Norddahl GL, Saemundsdottir J, Sigurdsson A, Sulem P, Agustsdottir AB, Eiriksdottir B, Fridriksdottir R, Gardarsdottir EE, Georgsson G, Gretarsdottir OS, Gudmundsson KR, Gunnarsdottir TR, Gylfason A, Holm H, Jensson BO, Jonasdottir A, Jonsson F, Josefsdottir KS, Kristjansson T, Magnusdottir DN, le Roux L, Sigmundsdottir G, Sveinbjornsson G, Sveinsdottir KE, Sveinsdottir M, Thorarensen EA, Thorbjornsson B, Löve A, Masson G, Jonsdottir I, Möller AD, Gudnason T, Kristinsson KG, Thorsteinsdottir U, Stefansson K. Spread of SARS-CoV-2 in the Icelandic Population. N Engl J Med. 2020;382:2302–2315. doi: 10.1056/NEJMoa2006100. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

