Infections in Infants with SCID: Isolation, Infection Screening, and Prophylaxis in PIDTC Centers
- PMID: 33006109
- PMCID: PMC8388237
- DOI: 10.1007/s10875-020-00865-9
Infections in Infants with SCID: Isolation, Infection Screening, and Prophylaxis in PIDTC Centers
Erratum in
-
Correction to: Infections in Infants with SCID: Isolation, Infection Screening and Prophylaxis in PIDTC Centers.J Clin Immunol. 2021 Feb;41(2):498-500. doi: 10.1007/s10875-020-00917-0. J Clin Immunol. 2021. PMID: 33274413 No abstract available.
Abstract
Purpose: The Primary Immune Deficiency Treatment Consortium (PIDTC) enrolled children with severe combined immunodeficiency (SCID) in a prospective natural history study of hematopoietic stem cell transplant (HSCT) outcomes over the last decade. Despite newborn screening (NBS) for SCID, infections occurred prior to HSCT. This study's objectives were to define the types and timing of infection prior to HSCT in patients diagnosed via NBS or by family history (FH) and to understand the breadth of strategies employed at PIDTC centers for infection prevention.
Methods: We analyzed retrospective data on infections and pre-transplant management in patients with SCID diagnosed by NBS and/or FH and treated with HSCT between 2010 and 2014. PIDTC centers were surveyed in 2018 to understand their practices and protocols for pre-HSCT management.
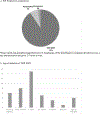
Results: Infections were more common in patients diagnosed via NBS (55%) versus those diagnosed via FH (19%) (p = 0.012). Outpatient versus inpatient management did not impact infections (47% vs 35%, respectively; p = 0.423). There was no consensus among PIDTC survey respondents as to the best setting (inpatient vs outpatient) for pre-HSCT management. While isolation practices varied, immunoglobulin replacement and antimicrobial prophylaxis were more uniformly implemented.
Conclusion: Infants with SCID diagnosed due to FH had lower rates of infection and proceeded to HSCT more quickly than did those diagnosed via NBS. Pre-HSCT management practices were highly variable between centers, although uses of prophylaxis and immunoglobulin support were more consistent. This study demonstrates a critical need for development of evidence-based guidelines for the pre-HSCT management of infants with SCID following an abnormal NBS.
Trial registration: NCT01186913.
Keywords: Infections; hematopoietic stem cell transplant; newborn screening; primary immunodeficiency; prophylaxis; severe combined immunodeficiency.
Conflict of interest statement
Figures

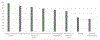


References
-
- Shearer WT, Dunn E, Notarangelo LD, Dvorak CC, Puck JM, Logan BR, et al.Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol. 2014;133(4):1092–8. - PMC - PubMed
-
- Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99(3):872–8. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

