Modulation of voltage-gated CaV2.2 Ca2+ channels by newly identified interaction partners
- PMID: 33006503
- PMCID: PMC7567506
- DOI: 10.1080/19336950.2020.1831328
Modulation of voltage-gated CaV2.2 Ca2+ channels by newly identified interaction partners
Abstract
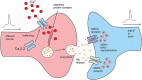
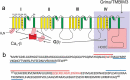
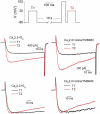
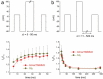
Voltage-gated Ca2+ channels are typically integrated in a complex network of protein-protein-interactions, also referred to as Ca2+ channel nanodomains. Amongst the neuronal CaV2 channel family, CaV2.2 is of particular importance due to its general role for signal transmission from the periphery to the central nervous system, but also due to its significance for pain perception. Thus, CaV2.2 is an ideal target candidate to search for pharmacological inhibitors but also for novel modulatory interactors. In this review we summarize the last years findings of our intense screenings and characterization of the six CaV2.2 interaction partners, tetraspanin-13 (TSPAN-13), reticulon 1 (RTN1), member 1 of solute carrier family 38 (SLC38), prostaglandin D2 synthase (PTGDS), transmembrane protein 223 (TMEM223), and transmembrane BAX inhibitor motif 3 (Grina/TMBIM3) containing protein. Each protein shows a unique way of channel modulation as shown by extensive electrophysiological studies. Amongst the newly identified interactors, Grina/TMBIM3 is most striking due to its modulatory effect which is rather comparable to G-protein regulation.
Keywords: Cav2.2; G protein; Grina/TMBIM3; calcium channel; channel modulation.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures
Similar articles
-
Four novel interaction partners demonstrate diverse modulatory effects on voltage-gated CaV2.2 Ca2+ channels.Pflugers Arch. 2019 Jun;471(6):861-874. doi: 10.1007/s00424-018-02248-x. Epub 2019 Jan 5. Pflugers Arch. 2019. PMID: 30612149
-
Grina/TMBIM3 modulates voltage-gated CaV2.2 Ca2+ channels in a G-protein-like manner.Cell Calcium. 2019 Jun;80:71-78. doi: 10.1016/j.ceca.2019.04.002. Epub 2019 Apr 8. Cell Calcium. 2019. PMID: 30991297
-
Tetraspanin-13 modulates voltage-gated CaV2.2 Ca2+ channels.Sci Rep. 2013;3:1777. doi: 10.1038/srep01777. Sci Rep. 2013. PMID: 23648579 Free PMC article.
-
Presynaptic calcium channels.Neurosci Res. 2018 Feb;127:33-44. doi: 10.1016/j.neures.2017.09.012. Epub 2018 Jan 6. Neurosci Res. 2018. PMID: 29317246 Review.
-
Presynaptic Calcium Channels.Int J Mol Sci. 2019 May 6;20(9):2217. doi: 10.3390/ijms20092217. Int J Mol Sci. 2019. PMID: 31064106 Free PMC article. Review.
Cited by
-
Identification and validation of key genes associated with atrial fibrillation in the elderly.Front Cardiovasc Med. 2023 Mar 29;10:1118686. doi: 10.3389/fcvm.2023.1118686. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37063972 Free PMC article.
References
-
- Ertel EA, Campbell KP, Harpold MM, et al. Nomenclature of voltage-gated calcium channels. Neuron. 2000. March;25(3):533–535. - PubMed
-
- Nowycky MC, Fox AP, Tsien RW.. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985. August 1-7;316(6027):440–443. - PubMed
-
- Westenbroek RE, Hell JW, Warner C, et al. Biochemical properties and subcellular distribution of an N-type calcium channel alpha 1 subunit. Neuron. 1992. December;9(6):1099–1115. - PubMed
-
- Altier C, Zamponi GW. Targeting Ca2+ channels to treat pain: T-type versus N-type. Trends Pharmacol Sci. 2004. September;25(9):465–470. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
