Optical Coherence Tomography Can Be Used to Assess Glaucomatous Optic Nerve Damage in Most Eyes With High Myopia
- PMID: 33006872
- PMCID: PMC7534586
- DOI: 10.1097/IJG.0000000000001631
Optical Coherence Tomography Can Be Used to Assess Glaucomatous Optic Nerve Damage in Most Eyes With High Myopia
Abstract
Precis: It is generally assumed that optical coherence tomography (OCT) cannot be used to diagnose glaucomatous optic neuropathy (GON) in high myopes. However, this study presents evidence that there is sufficient information in OCT scans to allow for accurate diagnosis of GON in most eyes with high myopia.
Purpose: The purpose of this study was to test the hypothesis that glaucomatous damage can be accurately diagnosed in most high myopes via an assessment of the OCT results.
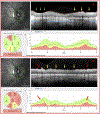
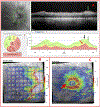
Patients and methods: One hundred eyes from 60 glaucoma patients or suspects, referred for OCT scans and evaluation, had corrected spherical refractive errors worse than -6 D and/or axial lengths ≥26.5 mm. An OCT specialist judged whether the eye had GON, based upon OCT circle scans of the disc and cube scans centered on the macula. A glaucoma specialist made the same judgement using all available information (eg, family history, repeat visits, intraocular pressure, 10-2 and 24-2 visual fields, OCT). A reference standard was created based upon the glaucoma specialist's classifications. In addition, the glaucoma specialist judged whether the eyes had peripapillary atrophy (PPA), epiretinal membrane (ERM), tilted disc (TD), and/or a paravascular inner retinal defect (PIRD).
Results: The OCT specialist correctly identified 97 of the 100 eyes using the OCT information. In 63% of the cases, the inner circle scan alone was sufficient. For the rest, additional scans were requested. In addition, 81% of the total eyes had: PPA (79%), ERM (18%), PIRD (26%), and/or TD (48%).
Conclusions: For most eyes with high myopia, there is sufficient information in OCT scans to allow for accurate diagnosis of GON. However, the optimal use of the OCT will depend upon training to read OCT scans, which includes taking into consideration myopia related OCT artifacts and segmentation errors, as well as PPA, ERM, PIRD, and TD.
Figures









References
-
- Weinreb RN, Leung CK, Crowston JG, et al. Primary open-angle glaucoma. Nat Rev Dis Primers. 2016;2:16067. - PubMed
-
- Henaine-Berra A, Zand-Hadas IM, Fromow-Guerra J, Garcia-Aguirre G. Prevalence of macular anatomic abnormalities in high myopia. Ophthalmic Surg Lasers Imaging Retina. 2013;44(2):140–144. - PubMed
-
- Liu W, Gong L, Li Y, Zhu X, Stewart JM, Wang C. Peripapillary Atrophy in High Myopia. Curr Eye Res. 2017;42(9):1308–1312. - PubMed
-
- Muraoka Y, Tsujikawa A, Hata M, et al. Paravascular inner retinal defect associated with high myopia or epiretinal membrane. JAMA Ophthalmol. 2015;133(4):413–420. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

