Copper-Catalyzed Propargylation of Nitroalkanes
- PMID: 33006901
- PMCID: PMC7579671
- DOI: 10.1021/acs.orglett.0c03061
Copper-Catalyzed Propargylation of Nitroalkanes
Abstract
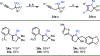
Using a commercially available, inexpensive, and abundant copper catalyst system, an efficient α-functionalization of nitroalkanes with propargyl bromides is now established. This mild and robust method is highly functional group tolerant and provides straightforward access to complex secondary and tertiary homopropargylic nitroalkanes. Moreover, the utility of these α-propargylated nitroalkanes is demonstrated through downstream functionalization to biologically relevant, five-membered N-heterocycles such as pyrroles and 2-pyrrolines.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Ono N, The Nitro Group in Organic Synthesis. Wiley-VCH: New York, 2001;
- Ballini R; Palmieri A, Synthetic Procedures for the Preparation of Nitroalkanes. Adv. Synth. Catal 2018, 360, 2240–2266.
-
- Aleksandrowicz P; Piotrowska H; Sas W, Palladium catalyzed C-allylation of nitroalkanes. Tetrahedron 1982, 38, 1321–1327;
- Maki K; Kanai M; Shibasaki M, Pd-Catalyzed Allylic Alkylation of Secondary Nitroalkanes. Tetrahedron 2007, 63, 4250–4257;
- Rieck H; Helmchen G, Palladium Complex Catalyzed Asymmetric Allylic Substitutions with Nitromethane: Enantioselectivities Exceeding 99.9%ee. Angew. Chem. Int. Ed. Engl 1995, 34, 2687–2689;
- VanGelder KF; Kozlowski MC, Palladium-Catalyzed α-Arylation of Aryl Nitromethanes. Org. Lett 2015, 17, 5748–5751; - PMC - PubMed
- Vogl EM; Buchwald SL, Palladium-catalyzed monoarylation of nitroalkanes. J Org Chem 2002, 67, 106–11; - PubMed
- Grenning AJ; Tunge JA, Deacylative Allylation of Nitroalkanes: Unsymmetric Bisallylation by a Three-Component Coupling. Angew. Chem. Int. Ed 2011, 50, 1688–1691; - PMC - PubMed
- Chang C-Y; Wu Y-K, Palladium-Catalyzed α-Allylation of Secondary Nitroalkanes with Allylic Alcohols and Strategic Exploitation of Seebach’s Reagent for the Total Synthesis of (±)-Adalinine. J. Org. Chem 2018, 83, 6217–6224; - PubMed
- Cristòfol À; Escudero-Adán EC; Kleij AW, Palladium-Catalyzed (Z)-Selective Allylation of Nitroalkanes: Access to Highly Functionalized Homoallylic Scaffolds. J. Org. Chem 2018, 83, 9978–9990; - PubMed
- Zhao C; Shah BH; Khan I; Kan Y; Zhang YJ, Enantioselective Synthesis of Isoxazoline N-Oxides via Pd-Catalyzed Asymmetric Allylic Cycloaddition of Nitro-Containing Allylic Carbonates. Org. Lett 2019, 21, 9045–9049. - PubMed
-
- Ballini R; Petrini M, Nitroalkanes as key building blocks for the synthesis of heterocyclic derivatives. Arkivoc 2009, 195–223.
-
- For a recent example see:
- Barber DM; Sanganee H; Dixon DJ, One-pot nitro-Mannich/hydroamination cascades for the direct synthesis of 2,5-disubstituted pyrroles using base and gold catalysis. Chem. Comm 2011, 47, 4379–4381. - PubMed
-
- Uraguchi D; Nakamura S; Ooi T, Catalytic Asymmetric Direct Henry Reaction of Ynals: Short Syntheses of (2S,3R)-(+)-Xestoaminol C and (−)-Codonopsinines. Angew. Chem. Int. Ed 2010, 49, 7562–7565; - PubMed
- Mei H; Xiao X; Zhao X; Fang B; Liu X; Lin L; Feng X, Catalytic Asymmetric Henry Reaction of Nitroalkanes and Aldehydes Catalyzed by a Chiral N,N′-Dioxide/Cu(I) Complex. J. Org. Chem 2015, 80, 2272–2280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

