The red flour beetle Tribolium castaneum: A model for host-microbiome interactions
- PMID: 33006995
- PMCID: PMC7531845
- DOI: 10.1371/journal.pone.0239051
The red flour beetle Tribolium castaneum: A model for host-microbiome interactions
Abstract
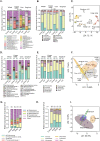
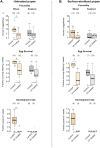
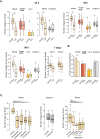
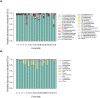
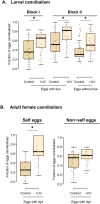
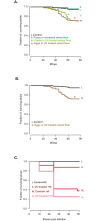
A large body of ongoing research focuses on understanding the mechanisms and processes underlying host-microbiome interactions, and predicting their ecological and evolutionary outcomes. To draw general conclusions about such interactions and understand how they are established, we must synthesize information from a diverse set of species. We analysed the microbiome of an important insect model-the red flour beetle Tribolium castaneum-which is a widespread generalist pest of stored cereals. The beetles complete their entire life cycle in flour, which thus serves multiple functions: habitat, food, and a source of microbes. We determined key factors that shape the T. castaneum microbiome, established protocols to manipulate it, and tested its consequences for host fitness. We show that the T. castaneum microbiome is derived from flour-acquired microbes, and varies as a function of (flour) resource and beetle density. Beetles gain multiple fitness benefits from their microbiome, such as higher fecundity, egg survival, and lifespan; and reduced cannibalism. In contrast, the microbiome has a limited effect on development rate, and does not enhance pathogen resistance. Importantly, the benefits are derived only from microbes in the ancestral resource (wheat flour), and not from novel resources such as finger millet, sorghum, and corn. Notably, the microbiome is not essential for beetle survival and development under any of the tested conditions. Thus, the red flour beetle is a tractable model system to understand the ecology, evolution and mechanisms of host-microbiome interactions, while closely mimicking the host species' natural niche.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

