Furin Inhibitors Block SARS-CoV-2 Spike Protein Cleavage to Suppress Virus Production and Cytopathic Effects
- PMID: 33007239
- PMCID: PMC7510585
- DOI: 10.1016/j.celrep.2020.108254
Furin Inhibitors Block SARS-CoV-2 Spike Protein Cleavage to Suppress Virus Production and Cytopathic Effects
Abstract
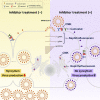
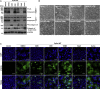
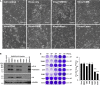
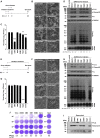
Development of specific antiviral agents is an urgent unmet need for SARS-coronavirus 2 (SARS-CoV-2) infection. This study focuses on host proteases that proteolytically activate the SARS-CoV-2 spike protein, critical for its fusion after binding to angiotensin-converting enzyme 2 (ACE2), as antiviral targets. We first validate cleavage at a putative furin substrate motif at SARS-CoV-2 spikes by expressing it in VeroE6 cells and find prominent syncytium formation. Cleavage and the syncytium are abolished by treatment with the furin inhibitors decanoyl-RVKR-chloromethylketone (CMK) and naphthofluorescein, but not by the transmembrane protease serine 2 (TMPRSS2) inhibitor camostat. CMK and naphthofluorescein show antiviral effects on SARS-CoV-2-infected cells by decreasing virus production and cytopathic effects. Further analysis reveals that, similar to camostat, CMK blocks virus entry, but it further suppresses cleavage of spikes and the syncytium. Naphthofluorescein acts primarily by suppressing viral RNA transcription. Therefore, furin inhibitors may be promising antiviral agents for prevention and treatment of SARS-CoV-2 infection.
Keywords: SARS-CoV-2; cytopathic effect; furin; spike; syncytium.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

