Passive Transfer of Vaccine-Elicited Antibodies Protects against SIV in Rhesus Macaques
- PMID: 33007262
- PMCID: PMC7534693
- DOI: 10.1016/j.cell.2020.08.033
Passive Transfer of Vaccine-Elicited Antibodies Protects against SIV in Rhesus Macaques
Abstract
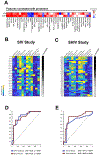
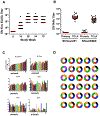
Several HIV-1 and SIV vaccine candidates have shown partial protection against viral challenges in rhesus macaques. However, the protective efficacy of vaccine-elicited polyclonal antibodies has not previously been demonstrated in adoptive transfer studies in nonhuman primates. In this study, we show that passive transfer of purified antibodies from vaccinated macaques can protect naive animals against SIVmac251 challenges. We vaccinated 30 rhesus macaques with Ad26-SIV Env/Gag/Pol and SIV Env gp140 protein vaccines and assessed the induction of antibody responses and a putative protective signature. This signature included multiple antibody functions and correlated with upregulation of interferon pathways in vaccinated animals. Adoptive transfer of purified immunoglobulin G (IgG) from the vaccinated animals with the most robust protective signatures provided partial protection against SIVmac251 challenges in naive recipient rhesus macaques. These data demonstrate the protective efficacy of purified vaccine-elicited antiviral antibodies in this model, even in the absence of virus neutralization.
Keywords: ADCC; Ad26; HIV; SIV; adoptive transfer; antibodies; systems biology; transcriptomics; vaccines.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests D.H.B. is a co-inventor on related HIV-1 vaccine patents. The authors otherwise declare no competing financial interests.
Figures
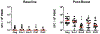
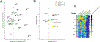



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

