Design and synthesis of novel methoxypyridine-derived gamma-secretase modulators
- PMID: 33007551
- PMCID: PMC7917164
- DOI: 10.1016/j.bmc.2020.115734
Design and synthesis of novel methoxypyridine-derived gamma-secretase modulators
Abstract
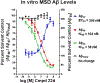
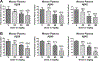
The evolution of gamma-secretase modulators (GSMs) through the introduction of novel heterocycles with the goal of aligning activity for reducing the levels of Aβ42 and properties consistent with a drug-like molecule are described. The insertion of a methoxypyridine motif within the tetracyclic scaffold provided compounds with improved activity for arresting Aβ42 production as well as improved properties, including solubility. In vivo pharmacokinetic analysis demonstrated that several compounds within the novel series were capable of crossing the BBB and accessing the therapeutic target. Treatment with methoxypyridine-derived compound 64 reduced Aβ42 levels in the plasma of J20 mice, in addition to reducing Aβ42 levels in the plasma and brain of Tg2576 mice.
Keywords: Alzheimer’s disease; Amyloid beta; Aβ42 reduction; Gamma secretase modulator; Methoxypyridines; Structure activity relationship.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
☒The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Drs. S. L. Wagner and R. E. Tanzi are shareholders and cofounders of a privately held company (Neurogenetic Pharmaceuticals, Inc.) that holds rights to a gamma-secretase modulator previously in clinical development.
Figures


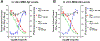

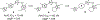
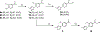

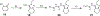
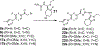
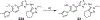
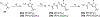

References
-
- Association, A. s., Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2019, 15 (3), 321–387.
-
- Glenner GG; Wong CW, Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 1984, 120 (3), 885–90. - PubMed
-
- Dickson DW, The Pathogenesis of Senile Plaques. Journal of Neuropathology & Experimental Neurology 1997, 56 (4), 321–339. - PubMed
-
- Rowe CC; Ellis KA; Rimajova M; Bourgeat P; Pike KE; Jones G; Fripp J; Tochon-Danguy H; Morandeau L; O’Keefe G; Price R; Raniga P; Robins P; Acosta O; Lenzo N; Szoeke C; Salvado O; Head R; Martins R; Masters CL; Ames D; Villemagne VL, Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 2010, 31 (8), 1275–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

