Identification of Plasma Glycosphingolipids as Potential Biomarkers for Prostate Cancer (PCa) Status
- PMID: 33007922
- PMCID: PMC7600119
- DOI: 10.3390/biom10101393
Identification of Plasma Glycosphingolipids as Potential Biomarkers for Prostate Cancer (PCa) Status
Abstract
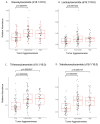
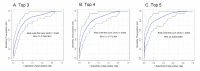
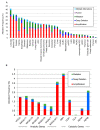
Prostate cancer (PCa) is the most common male cancer and the second leading cause of cancer death in United States men. Controversy continues over the effectiveness of prostate-specific antigen (PSA) for distinguishing aggressive from indolent PCa. There is a critical need for more specific and sensitive biomarkers to detect and distinguish low- versus high-risk PCa cases. Discovery metabolomics were performed utilizing ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS) on plasma samples from 159 men with treatment naïve prostate cancer participating in the North Carolina-Louisiana PCa Project to determine if there were metabolites associated with aggressive PCa. Thirty-five identifiable plasma small molecules were associated with PCa aggressiveness, 15 of which were sphingolipids; nine common molecules were present in both African-American and European-American men. The molecules most associated with PCa aggressiveness were glycosphingolipids; levels of trihexosylceramide and tetrahexosylceramide were most closely associated with high-aggressive PCa. The Cancer Genome Atlas was queried to determine gene alterations within glycosphingolipid metabolism that are associated with PCa and other cancers. Genes that encode enzymes associated with the metabolism of glycosphingolipids were altered in 12% of PCa and >30% of lung, uterine, and ovarian cancers. These data suggest that the identified plasma (glyco)sphingolipids should be further validated for their association with aggressive PCa, suggesting that specific sphingolipids may be included in a diagnostic signature for PCa.
Keywords: biomarker; ceramide; lipidomic; metabolomic; sphingolipid.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures



References
-
- Martin R.M., Donovan J.L., Turner E.L., Metcalfe C., Young G.J., Walsh E.I., Lane J.A., Noble S., Oliver S.E., Evans S., et al. Effect of a Low-Intensity PSA-Based Screening Intervention on Prostate Cancer Mortality: The CAP Randomized Clinical Trial. JAMA. 2018;319:883–895. doi: 10.1001/jama.2018.0154. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

