Role of Viral Ribonucleoproteins in Human Papillomavirus Type 16 Gene Expression
- PMID: 33007936
- PMCID: PMC7600041
- DOI: 10.3390/v12101110
Role of Viral Ribonucleoproteins in Human Papillomavirus Type 16 Gene Expression
Abstract
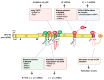
Human papillomaviruses (HPVs) depend on the cellular RNA-processing machineries including alternative RNA splicing and polyadenylation to coordinate HPV gene expression. HPV RNA processing is controlled by cis-regulatory RNA elements and trans-regulatory factors since the HPV splice sites are suboptimal. The definition of HPV exons and introns may differ between individual HPV mRNA species and is complicated by the fact that many HPV protein-coding sequences overlap. The formation of HPV ribonucleoproteins consisting of HPV pre-mRNAs and multiple cellular RNA-binding proteins may result in the different outcomes of HPV gene expression, which contributes to the HPV life cycle progression and HPV-associated cancer development. In this review, we summarize the regulation of HPV16 gene expression at the level of RNA processing with focus on the interactions between HPV16 pre-mRNAs and cellular RNA-binding factors.
Keywords: SR proteins; hnRNP; human papillomavirus (HPV); papillomavirus; polyadenylation; splicing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Howley P.M., Lowy D.R. Papillomaviridae. In: Knipe D.M., Howley P.M., editors. Virology. 5th ed. Volume 2. Lippincott/The Williams & Wilkins Co.; Philadelphia, PA, USA: 2006. pp. 2299–2354.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

