Mixed Amphiphilic Polymeric Nanoparticles of Chitosan, Poly(vinyl alcohol) and Poly(methyl methacrylate) for Intranasal Drug Delivery: A Preliminary In Vivo Study
- PMID: 33008001
- PMCID: PMC7582691
- DOI: 10.3390/molecules25194496
Mixed Amphiphilic Polymeric Nanoparticles of Chitosan, Poly(vinyl alcohol) and Poly(methyl methacrylate) for Intranasal Drug Delivery: A Preliminary In Vivo Study
Abstract
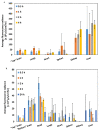
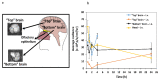
Intranasal (i.n.) administration became an alternative strategy to bypass the blood-brain barrier and improve drug bioavailability in the brain. The main goal of this work was to preliminarily study the biodistribution of mixed amphiphilic mucoadhesive nanoparticles made of chitosan-g-poly(methyl methacrylate) and poly(vinyl alcohol)-g-poly(methyl methacrylate) and ionotropically crosslinked with sodium tripolyphosphate in the brain after intravenous (i.v.) and i.n. administration to Hsd:ICR mice. After i.v. administration, the highest nanoparticle accumulation was detected in the liver, among other peripheral organs. After i.n. administration of a 10-times smaller nanoparticle dose, the accumulation of the nanoparticles in off-target organs was much lower than after i.v. injection. In particular, the accumulation of the nanoparticles in the liver was 20 times lower than by i.v. When brains were analyzed separately, intravenously administered nanoparticles accumulated mainly in the "top" brain, reaching a maximum after 1 h. Conversely, in i.n. administration, nanoparticles were detected in the "bottom" brain and the head (maximum reached after 2 h) owing to their retention in the nasal mucosa and could serve as a reservoir from which the drug is released and transported to the brain over time. Overall, results indicate that i.n. nanoparticles reach similar brain bioavailability, though with a 10-fold smaller dose, and accumulate in off-target organs to a more limited extent and only after redistribution through the systemic circulation. At the same time, both administration routes seem to lead to differential accumulation in brain regions, and thus, they could be beneficial in the treatment of different medical conditions.
Keywords: biodistribution; blood–brain barrier (BBB); central nervous system (CNS); intranasal delivery; self-assembled polymeric nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Mixed Mucoadhesive Amphiphilic Polymeric Nanoparticles Cross a Model of Nasal Septum Epithelium in Vitro.ACS Appl Mater Interfaces. 2019 Jun 19;11(24):21360-21371. doi: 10.1021/acsami.9b04766. Epub 2019 Jun 10. ACS Appl Mater Interfaces. 2019. PMID: 31124655
-
"Application of Box-Behnken design for optimization and development of quetiapine fumarate loaded chitosan nanoparticles for brain delivery via intranasal route* ".Int J Biol Macromol. 2016 Aug;89:206-18. doi: 10.1016/j.ijbiomac.2016.04.076. Epub 2016 Apr 27. Int J Biol Macromol. 2016. PMID: 27130654
-
Nose to brain delivery in rats: Effect of surface charge of rhodamine B labeled nanocarriers on brain subregion localization.Colloids Surf B Biointerfaces. 2017 Jun 1;154:297-306. doi: 10.1016/j.colsurfb.2017.03.035. Epub 2017 Mar 18. Colloids Surf B Biointerfaces. 2017. PMID: 28363190
-
Intranasal Lipid Particulate Drug Delivery Systems: An Update on Clinical Challenges and Biodistribution Studies of Cerebroactive Drugs in Alzheimer's disease.Curr Pharm Des. 2020;26(27):3281-3299. doi: 10.2174/1381612826666200331085854. Curr Pharm Des. 2020. PMID: 32228421 Review.
-
Chitosan-based nanoparticles for tumor-targeted drug delivery.Int J Biol Macromol. 2015 Jan;72:1313-22. doi: 10.1016/j.ijbiomac.2014.10.052. Epub 2014 Nov 3. Int J Biol Macromol. 2015. PMID: 25450550 Review.
Cited by
-
Effects of miR-214 on adenosine A2A receptor and carboxymethyl chitosan nanoparticles on the function of keloid fibroblasts and their mechanisms.Sci Rep. 2024 Feb 28;14(1):4896. doi: 10.1038/s41598-024-54125-6. Sci Rep. 2024. PMID: 38418830 Free PMC article.
-
Biomaterial strategies for regulating the neuroinflammatory response.Mater Adv. 2024 Apr 10;5(10):4025-4054. doi: 10.1039/d3ma00736g. eCollection 2024 May 20. Mater Adv. 2024. PMID: 38774837 Free PMC article. Review.
-
Heterocellular spheroids of the neurovascular blood-brain barrier as a platform for personalized nanoneuromedicine.iScience. 2021 Feb 12;24(3):102183. doi: 10.1016/j.isci.2021.102183. eCollection 2021 Mar 19. iScience. 2021. PMID: 33718835 Free PMC article.
-
Functionalized Nanomaterials as Tailored Theranostic Agents in Brain Imaging.Nanomaterials (Basel). 2021 Dec 22;12(1):18. doi: 10.3390/nano12010018. Nanomaterials (Basel). 2021. PMID: 35009968 Free PMC article. Review.
-
Targeting Systems to the Brain Obtained by Merging Prodrugs, Nanoparticles, and Nasal Administration.Pharmaceutics. 2021 Jul 27;13(8):1144. doi: 10.3390/pharmaceutics13081144. Pharmaceutics. 2021. PMID: 34452105 Free PMC article. Review.
References
-
- Blanco-Prieto M. Drug Delivery to the Central Nervous System: A Review. J. Pharm. Sci. 2003;6:252–273. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

