A Genotype-Phenotype Study of High-Resolution FMR1 Nucleic Acid and Protein Analyses in Fragile X Patients with Neurobehavioral Assessments
- PMID: 33008014
- PMCID: PMC7601415
- DOI: 10.3390/brainsci10100694
A Genotype-Phenotype Study of High-Resolution FMR1 Nucleic Acid and Protein Analyses in Fragile X Patients with Neurobehavioral Assessments
Abstract
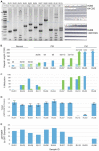
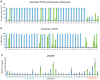
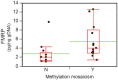
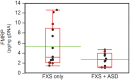
Fragile X syndrome (FXS) is caused by silencing of the FMR1 gene, which encodes a protein with a critical role in synaptic plasticity. The molecular abnormality underlying FMR1 silencing, CGG repeat expansion, is well characterized; however, delineation of the pathway from DNA to RNA to protein using biosamples from well characterized patients with FXS is limited. Since FXS is a common and prototypical genetic disorder associated with intellectual disability (ID) and autism spectrum disorder (ASD), a comprehensive assessment of the FMR1 DNA-RNA-protein pathway and its correlations with the neurobehavioral phenotype is a priority. We applied nine sensitive and quantitative assays evaluating FMR1 DNA, RNA, and FMRP parameters to a reference set of cell lines representing the range of FMR1 expansions. We then used the most informative of these assays on blood and buccal specimens from cohorts of patients with different FMR1 expansions, with emphasis on those with FXS (N = 42 total, N = 31 with FMRP measurements). The group with FMRP data was also evaluated comprehensively in terms of its neurobehavioral profile, which allowed molecular-neurobehavioral correlations. FMR1 CGG repeat expansions, methylation levels, and FMRP levels, in both cell lines and blood samples, were consistent with findings of previous FMR1 genomic and protein studies. They also demonstrated a high level of agreement between blood and buccal specimens. These assays further corroborated previous reports of the relatively high prevalence of methylation mosaicism (slightly over 50% of the samples). Molecular-neurobehavioral correlations confirmed the inverse relationship between overall severity of the FXS phenotype and decrease in FMRP levels (N = 26 males, mean 4.2 ± 3.3 pg FMRP/ng genomic DNA). Other intriguing findings included a significant relationship between the diagnosis of FXS with ASD and two-fold lower levels of FMRP (mean 2.8 ± 1.3 pg FMRP/ng genomic DNA, p = 0.04), in particular observed in younger age- and IQ-adjusted males (mean age 6.9 ± 0.9 years with mean 3.2 ± 1.2 pg FMRP/ng genomic DNA, 57% with severe ASD), compared to FXS without ASD. Those with severe ID had even lower FMRP levels independent of ASD status in the male-only subset. The results underscore the link between FMR1 expansion, gene methylation, and FMRP deficit. The association between FMRP deficiency and overall severity of the neurobehavioral phenotype invites follow up studies in larger patient cohorts. They would be valuable to confirm and potentially extend our initial findings of the relationship between ASD and other neurobehavioral features and the magnitude of FMRP deficit. Molecular profiling of individuals with FXS may have important implications in research and clinical practice.
Keywords: FMR1; FMRP; PCR; autism spectrum disorder; fragile X syndrome.
Conflict of interest statement
D.B.B. has received funding from Seaside, Roche, Neuren, Pfizer, Shire, Lundbeck, Forest, Sunovion, SyneuRX, Alcobra, Akili, Medgenics, Purdue, Supernus as a main sub-investigator, and Ovid and Zynerba Pharmaceuticals as the principal investigator on clinical trials. He also consulted on clinical trial outcome measures focused meetings (Seaside, Ovid). All the above funding has been directed to Kennedy Krieger Institute/the Johns Hopkins Medical Institutions; D.B.B. receives no personal funds, and the Institute has no relevant financial interest in any of the commercial entities listed. A.S., S.F.-S., E.B., K.N., K.C., K.J., J.K., A.H., and G.J.L. are or were employed by Asuragen at the time the work was performed and have or may have stock options in the company. G.J.L., E.B., and A.H. are inventors on one or more patents or patent applications associated with genotyping or epityping
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

