NMR Studies of Retroviral Genome Packaging
- PMID: 33008123
- PMCID: PMC7599994
- DOI: 10.3390/v12101115
NMR Studies of Retroviral Genome Packaging
Erratum in
-
Correction: Boyd, P.S., et al. NMR Studies of Retroviral Genome Packaging. Viruses 2020, 12, 1115.Viruses. 2021 Mar 11;13(3):453. doi: 10.3390/v13030453. Viruses. 2021. PMID: 33799918 Free PMC article.
Abstract
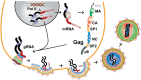
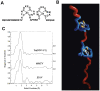
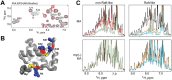
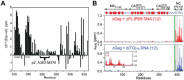
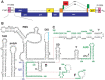
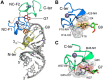
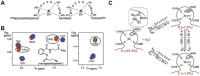
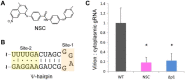
Nearly all retroviruses selectively package two copies of their unspliced RNA genomes from a cellular milieu that contains a substantial excess of non-viral and spliced viral RNAs. Over the past four decades, combinations of genetic experiments, phylogenetic analyses, nucleotide accessibility mapping, in silico RNA structure predictions, and biophysical experiments were employed to understand how retroviral genomes are selected for packaging. Genetic studies provided early clues regarding the protein and RNA elements required for packaging, and nucleotide accessibility mapping experiments provided insights into the secondary structures of functionally important elements in the genome. Three-dimensional structural determinants of packaging were primarily derived by nuclear magnetic resonance (NMR) spectroscopy. A key advantage of NMR, relative to other methods for determining biomolecular structure (such as X-ray crystallography), is that it is well suited for studies of conformationally dynamic and heterogeneous systems-a hallmark of the retrovirus packaging machinery. Here, we review advances in understanding of the structures, dynamics, and interactions of the proteins and RNA elements involved in retroviral genome selection and packaging that are facilitated by NMR.
Keywords: NMR; RNA; SAXS; cyro-EM; genome; packaging; protein; retrovirus; structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



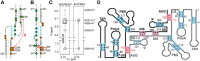


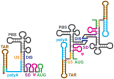





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

