Cerebrospinal fluid A beta 1-40 peptides increase in Alzheimer's disease and are highly correlated with phospho-tau in control individuals
- PMID: 33008460
- PMCID: PMC7532565
- DOI: 10.1186/s13195-020-00696-1
Cerebrospinal fluid A beta 1-40 peptides increase in Alzheimer's disease and are highly correlated with phospho-tau in control individuals
Abstract
Background: Amyloid pathology, which is one of the characteristics of Alzheimer's disease (AD), results from altered metabolism of the beta-amyloid (Aβ) peptide in terms of synthesis, clearance, or aggregation. A decrease in cerebrospinal fluid (CSF) level Aβ1-42 is evident in AD, and the CSF ratio Aβ42/Aβ40 has recently been identified as one of the most reliable diagnostic biomarkers of amyloid pathology. Variations in inter-individual levels of Aβ1-40 in the CSF have been observed in the past, but their origins remain unclear. In addition, the variation of Aβ40 in the context of AD studied in several studies has yielded conflicting results.
Methods: Here, we analyzed the levels of Aβ1-40 using multicenter data obtained on 2466 samples from six different cohorts in which CSF was collected under standardized protocols, centrifugation, and storage conditions. Tau and p-tau (181) concentrations were measured using commercially available in vitro diagnostic immunoassays. Concentrations of CSF Aβ1-42 and Aβ1-40 were measured by ELISA, xMAP technology, chemiluminescence immunoassay (CLIA), and mass spectrometry. Statistical analyses were calculated for parametric and non-parametric comparisons, linear regression, correlation, and odds ratios. The statistical tests were adjusted for the effects of covariates (age, in particular).
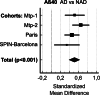
Results: Regardless of the analysis method used and the cohorts, a slight but significant age-independent increase in the levels of Aβ40 in CSF was observed in AD. We also found a strong positive correlation between the levels of Aβ1-40 and p-tau (181) in CSF, particularly in control patients.
Conclusions: These results indicate that an increase in the baseline level of amyloid peptides, which are associated with an increase in p-tau (181), may be a biological characteristic and possibly a risk factor for AD. Further studies will be needed to establish a causal link between increased baseline levels of Aβ40 and the development of the disease.
Keywords: Alzheimer’s disease; Amyloid peptides; Biomarkers; Cerebrospinal fluid (CSF); Tau proteins.
Conflict of interest statement
The authors report no conflict of interest to disclose.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

