β-glucan attenuates cognitive impairment via the gut-brain axis in diet-induced obese mice
- PMID: 33008466
- PMCID: PMC7532656
- DOI: 10.1186/s40168-020-00920-y
β-glucan attenuates cognitive impairment via the gut-brain axis in diet-induced obese mice
Abstract
Background: "Western" style dietary patterns are characterized by a high proportion of highly processed foods rich in fat and low in fiber. This diet pattern is associated with a myriad of metabolic dysfunctions, including neuroinflammation and cognitive impairment. β-glucan, the major soluble fiber in oat and barley grains, is fermented in the lower gastrointestinal tract, potentially impacting the microbial ecosystem and thus may improve elements of cognition and brain function via the gut-brain axis. The present study aimed to evaluate the effect of β-glucan on the microbiota gut-brain axis and cognitive function in an obese mouse model induced by a high-fat and fiber-deficient diet (HFFD).
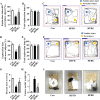
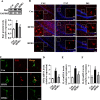
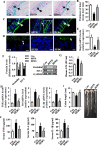
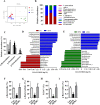
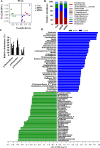
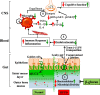
Results: After long-term supplementation for 15 weeks, β-glucan prevented HFFD-induced cognitive impairment assessed behaviorally by object location, novel object recognition, and nesting building tests. In the hippocampus, β-glucan countered the HFFD-induced microglia activation and its engulfment of synaptic puncta, and upregulation of proinflammatory cytokine (TNF-α, IL-1β, and IL-6) mRNA expression. Also, in the hippocampus, β-glucan significantly promoted PTP1B-IRS-pAKT-pGSK3β-pTau signaling for synaptogenesis, improved the synaptic ultrastructure examined by transmission electron microscopy, and increased both pre- and postsynaptic protein levels compared to the HFFD-treated group. In the colon, β-glucan reversed HFFD-induced gut barrier dysfunction increased the thickness of colonic mucus (Alcian blue and mucin-2 glycoprotein immunofluorescence staining), increased the levels of tight junction proteins occludin and zonula occludens-1, and attenuated bacterial endotoxin translocation. The HFFD resulted in microbiota alteration, effects abrogated by long-term β-glucan supplementation, with the β-glucan effects on Bacteroidetes and its lower taxa particularly striking. Importantly, the study of short-term β-glucan supplementation for 7 days demonstrated pronounced, rapid differentiating microbiota changes before the cognitive improvement, suggesting the possible causality of gut microbiota profile on cognition. In support, broad-spectrum antibiotic intervention abrogated β-glucan's effects on improving cognition, highlighting the role of gut microbiota to mediate cognitive behavior.
Conclusion: This study provides the first evidence that β-glucan improves indices of cognition and brain function with major beneficial effects all along the gut microbiota-brain axis. Our data suggest that elevating consumption of β-glucan-rich foods is an easily implementable nutritional strategy to alleviate detrimental features of gut-brain dysregulation and prevent neurodegenerative diseases associated with Westernized dietary patterns. Video Abstract.
Keywords: Cognition; Gut microbiota; Gut-brain axis; Obesity; β-glucan.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

