Burkholderia thailandensis Methylated Hydroxyalkylquinolines: Biosynthesis and Antimicrobial Activity in Cocultures
- PMID: 33008823
- PMCID: PMC7688213
- DOI: 10.1128/AEM.01452-20
Burkholderia thailandensis Methylated Hydroxyalkylquinolines: Biosynthesis and Antimicrobial Activity in Cocultures
Abstract
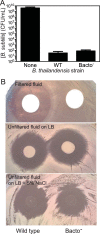
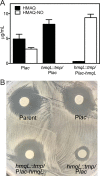
The bacterium Burkholderia thailandensis produces an arsenal of secondary metabolites that have diverse structures and roles in the ecology of this soil-dwelling bacterium. In coculture experiments, B. thailandensis strain E264 secretes an antimicrobial that nearly eliminates another soil bacterium, Bacillus subtilis strain 168. To identify the antimicrobial, we used a transposon mutagenesis approach. This screen identified antimicrobial-defective mutants with insertions in the hmqA, hmqC, and hmqF genes involved in biosynthesis of a family of 2-alkyl-4(1H)-quinolones called 4-hydroxy-3-methyl-2-alkenylquinolines (HMAQs), which are closely related to the Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs). Insertions also occurred in the previously uncharacterized gene BTH_II1576 ("hmqL"). The results confirm that BTH_II1576 is involved in generating N-oxide derivatives of HMAQs (HMAQ-NOs). Synthetic HMAQ-NO is active against B. subtilis 168, showing ∼50-fold more activity than HMAQ. Both the methyl group and the length of the carbon side chain account for the high activity of HMAQ-NO. The results provide new information on the biosynthesis and activities of HMAQs and reveal new insight into how these molecules might be important for the ecology of B. thailandensisIMPORTANCE The soil bacterium Burkholderia thailandensis produces 2-alkyl-4(1H)-quinolones that are mostly methylated 4-hydroxyalkenylquinolines, a family of relatively unstudied metabolites similar to molecules also synthesized by Pseudomonas aeruginosa Several of the methylated 4-hydroxyalkenylquinolines have antimicrobial activity against other species. We show that Bacillus subtilis strain 168 is particularly susceptible to N-oxidated methylalkenylquinolines (HMAQ-NOs). We confirmed that HMAQ-NO biosynthesis requires the previously unstudied protein HmqL. These results provide new information about the biology of 2-alkyl-4(1H)-quinolones, particularly the methylated 4-hydroxyalkenylquinolines, which are unique to B. thailandensis This study also has importance for understanding B. thailandensis secondary metabolites and has implications for potential therapeutic development.
Keywords: Burkholderia; cell-cell interaction; natural antimicrobial products; quinolones.
Copyright © 2020 American Society for Microbiology.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

