Activation-Induced Marker Expression Identifies Mycobacterium tuberculosis-Specific CD4 T Cells in a Cytokine-Independent Manner in HIV-Infected Individuals with Latent Tuberculosis
- PMID: 33008839
- PMCID: PMC7585460
- DOI: 10.4049/immunohorizons.2000051
Activation-Induced Marker Expression Identifies Mycobacterium tuberculosis-Specific CD4 T Cells in a Cytokine-Independent Manner in HIV-Infected Individuals with Latent Tuberculosis
Abstract
HIV infection is a significant risk factor for reactivation of latent Mycobacterium tuberculosis infection (LTBI) and progression to active tuberculosis disease, yet the mechanisms whereby HIV impairs T cell immunity to M. tuberculosis have not been fully defined. Evaluation of M. tuberculosis-specific CD4 T cells is commonly based on IFN-γ production, yet increasing evidence indicates the immune response to M. tuberculosis is heterogeneous and encompasses IFN-γ-independent responses. We hypothesized that upregulation of surface activation-induced markers (AIM) would facilitate detection of human M. tuberculosis-specific CD4 T cells in a cytokine-independent manner in HIV-infected and HIV-uninfected individuals with LTBI. PBMCs from HIV-infected and HIV-uninfected adults in Kenya were stimulated with CFP-10 and ESAT-6 peptides and evaluated by flow cytometry for upregulation of the activation markers CD25, OX40, CD69, and CD40L. Although M. tuberculosis-specific IFN-γ and IL-2 production was dampened in HIV-infected individuals, M. tuberculosis-specific CD25+OX40+ and CD69+CD40L+ CD4 T cells were detectable in the AIM assay in both HIV-uninfected and HIV-infected individuals with LTBI. Importantly, the frequency of M. tuberculosis-specific AIM+ CD4 T cells was not directly impacted by HIV viral load or CD4 count, thus demonstrating the feasibility of AIM assays for analysis of M. tuberculosis-specific CD4 T cells across a spectrum of HIV infection states. These data indicate that AIM assays enable identification of M. tuberculosis-specific CD4 T cells in a cytokine-independent manner in HIV-uninfected and HIV-infected individuals with LTBI in a high-tuberculosis burden setting, thus facilitating studies to define novel T cell correlates of protection to M. tuberculosis and elucidate mechanisms of HIV-associated dysregulation of antimycobacterial immunity.
Copyright © 2020 The Authors.
Conflict of interest statement
DISCLOSURES
The authors have no financial conflicts of interest.
Figures

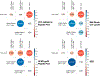


References
-
- World Health Organization. 2019. Global Tuberculosis Report 2019. World Health Organization, Geneva, Switzerland.
-
- Lawn SD, and Zumla AI. 2011. Tuberculosis. Lancet 378: 57–72. - PubMed
-
- Day CL, Abrahams DA, Harris LD, van Rooyen M, Stone L, de Kock M, and Hanekom WA. 2017. HIV-1 infection is associated with depletion and functional impairment of Mycobacterium tuberculosis-specific CD4 T cells in individuals with latent tuberculosis infection. J. Immunol 199: 2069–2080. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

