Integrative glycoproteomics reveals protein N-glycosylation aberrations and glycoproteomic network alterations in Alzheimer's disease
- PMID: 33008897
- PMCID: PMC7852392
- DOI: 10.1126/sciadv.abc5802
Integrative glycoproteomics reveals protein N-glycosylation aberrations and glycoproteomic network alterations in Alzheimer's disease
Abstract
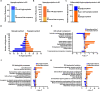
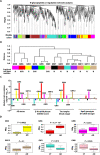
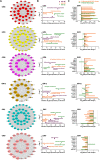
Protein N-glycosylation plays critical roles in controlling brain function, but little is known about human brain N-glycoproteome and its alterations in Alzheimer's disease (AD). Here, we report the first, large-scale, site-specific N-glycoproteome profiling study of human AD and control brains using mass spectrometry-based quantitative N-glycoproteomics. The study provided a system-level view of human brain N-glycoproteins and in vivo N-glycosylation sites and identified disease signatures of altered N-glycopeptides, N-glycoproteins, and N-glycosylation site occupancy in AD. Glycoproteomics-driven network analysis showed 13 modules of co-regulated N-glycopeptides/glycoproteins, 6 of which are associated with AD phenotypes. Our analyses revealed multiple dysregulated N-glycosylation-affected processes and pathways in AD brain, including extracellular matrix dysfunction, neuroinflammation, synaptic dysfunction, cell adhesion alteration, lysosomal dysfunction, endocytic trafficking dysregulation, endoplasmic reticulum dysfunction, and cell signaling dysregulation. Our findings highlight the involvement of N-glycosylation aberrations in AD pathogenesis and provide new molecular and system-level insights for understanding and treating AD.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- Scheltens P., Blennow K., Breteler M. M., de Strooper B., Frisoni G. B., Salloway S., Van der Flier W. M., Alzheimer's disease. Lancet 388, 505–517 (2016). - PubMed
-
- Soejitno A., Tjan A., Purwata T. E., Alzheimer's disease: Lessons learned from amyloidocentric clinical trials. CNS Drugs 29, 487–502 (2015). - PubMed
-
- Narayanan M., Huynh J. L., Wang K., Yang X., Yoo S., McElwee J., Zhang B., Zhang C., Lamb J. R., Xie T., Suver C., Molony C., Melquist S., Johnson A. D., Fan G., Stone D. J., Schadt E. E., Casaccia P., Emilsson V., Zhu J., Common dysregulation network in the human prefrontal cortex underlies two neurodegenerative diseases. Mol. Syst. Biol. 10, 743 (2014). - PMC - PubMed
-
- Sancesario G. M., Bernardini S., Alzheimer's disease in the omics era. Clin. Biochem. 59, 9–16 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

