Amlodipine/valsartan fixed-dose combination treatment in the management of hypertension: A double-blind, randomized trial
- PMID: 33009241
- PMCID: PMC7526577
- DOI: 10.1097/JCMA.0000000000000386
Amlodipine/valsartan fixed-dose combination treatment in the management of hypertension: A double-blind, randomized trial
Abstract
Background: To compare the fixed-dose combination (FDC) of amlodipine/valsartan 5/80 mg with valsartan 160 mg monotherapy for efficacy and safety in hypertensive patients.
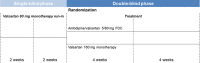
Methods: We designed this double-blind, randomized, and noninferiority trial in which patients with elevated systolic blood pressure (SBP) and/or diastolic blood pressure (DBP) were randomly assigned to receive amlodipine/valsartan 5/80 mg FDC or valsartan 160 mg monotherapy for 8 weeks. The primary endpoint was changes in office SBP and DBP from baseline to 8 weeks. Twenty-four-hour blood pressure (BP) and the incidence of adverse events were recorded.
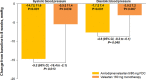
Results: A total of 42 patients underwent randomization. At 8 weeks, office SBP changes were -16.5 ± 15.5 mmHg (p < 0.001) with amlodipine/valsartan 5/80 mg FDC and -6.9 ± 11.4 mmHg (p = 0.012) with valsartan 160 mg monotherapy while corresponding changes in office DBP were -9.8 ± 7.7 mmHg (p < 0.001) and -2.5 ± 6.6 mmHg (p = 0.095), respectively. The between-group differences were -9.6 mmHg (95% CI, -18.1 to -1.1; p = 0.028) for SBP and -7.3 mmHg (95% CI, -11.8 to -2.8; p = 0.002) for DBP. Furthermore, reductions in both 24-hour SBP (-9.2 mmHg; 95% CI, -16.4 to -2.1; p = 0.013) and DBP (-4.6 mmHg; 95% CI, -9.2 to -0.1; p = 0.048) were consistently greater with amlodipine/valsartan 5/80 mg FDC than with valsartan 160 mg. Overall, 27 and 23 adverse events occurred in the amlodipine/valsartan 5/80 mg FDC group and in the valsartan 160 mg monotherapy group, respectively. The majority were mild and were not related to study medications. There were no significant differences in safety between two treatments.
Conclusion: Efficacy of amlodipine/valsartan 5/80 mg FDC was superior to that of valsartan 160 mg monotherapy while both treatments were well-tolerated.
Conflict of interest statement
Conflicts of interest: Dr. Wang reports honoraria from Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Novartis, and Pfizer. Dr. Chiang has been on the speaker bureau for AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Merck Sharp & Dohme, Novartis, Pfizer, Sanofi, and Servier. The other authors declare that they have no conflicts of interest related to the subject matter or materials discussed in this article.
Figures

Similar articles
-
Efficacy and tolerability of amlodipine/valsartan combination therapy in hypertensive patients not adequately controlled on valsartan monotherapy.Curr Med Res Opin. 2009 Feb;25(2):315-24. doi: 10.1185/03007990802630588. Curr Med Res Opin. 2009. PMID: 19192976 Clinical Trial.
-
Efficacy and tolerability of combination therapy with valsartan plus hydrochlorothiazide compared with amlodipine monotherapy in hypertensive patients with other cardiovascular risk factors: the VAST study.Clin Ther. 2005 May;27(5):578-87. doi: 10.1016/j.clinthera.2005.05.006. Clin Ther. 2005. PMID: 15978306 Clinical Trial.
-
A multicenter, randomized, and double-blind phase IV clinical trial to compare the efficacy and safety of fixed-dose combinations of amlodipine orotate/valsartan 5/160 mg versus valsartan/hydrochlorothiazide 160/12.5 mg in patients with essential hypertension uncontrolled by valsartan 160 mg monotherapy.Medicine (Baltimore). 2018 Sep;97(37):e12329. doi: 10.1097/MD.0000000000012329. Medicine (Baltimore). 2018. PMID: 30212981 Free PMC article. Clinical Trial.
-
Antihypertensive efficacy of olmesartan medoxomil or valsartan in combination with amlodipine: a review of factorial-design studies.Curr Med Res Opin. 2009 Jan;25(1):177-85. doi: 10.1185/03007990802597456. Curr Med Res Opin. 2009. PMID: 19210150 Review.
-
Amlodipine/valsartan single-pill combination: a review of its use in the management of hypertension.Am J Cardiovasc Drugs. 2009;9(5):309-30. doi: 10.2165/11201120-000000000-00000. Am J Cardiovasc Drugs. 2009. PMID: 19791840 Review.
Cited by
-
The effects of Olmesartan/amlodipine administered in the Morning or At Night on nocturnal blood pressure reduction in Chinese patients with mild-moderate essential hypertension (OMAN Trial): study protocol for a prospective, multicenter, randomized, open-label clinical trial {1}.Trials. 2023 Nov 28;24(1):770. doi: 10.1186/s13063-023-07726-x. Trials. 2023. PMID: 38017457 Free PMC article.
-
Blood ozonization in patients with mild to moderate COVID-19 pneumonia: a single centre experience.Intern Emerg Med. 2021 Apr;16(3):669-675. doi: 10.1007/s11739-020-02542-6. Epub 2020 Nov 1. Intern Emerg Med. 2021. PMID: 33131033 Free PMC article.
-
Two-Drug Combinations Therapy of Different Doses of Valsartan Existing Diverse Significance for Hypertensive Patients.Rev Cardiovasc Med. 2023 Jun 29;24(7):187. doi: 10.31083/j.rcm2407187. eCollection 2023 Jul. Rev Cardiovasc Med. 2023. PMID: 39077003 Free PMC article.
References
-
- Sundström J, Arima H, Jackson R, Turnbull F, Rahimi K, Chalmers J, et al. ; Blood Pressure Lowering Treatment Trialists’ Collaboration Effects of blood pressure reduction in mild hypertension: a systematic review and meta-analysis. Ann Intern Med. 2015;162:184–91. - PubMed
-
- Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, et al. ; ALLHAT Collaborative Research Group Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich). 2002;4:393–404. - PubMed
-
- Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, et al. ; ASCOT Investigators Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

