Acute Radiation-induced Lung Injury in the Non-human Primate: A Review and Comparison of Mortality and Co-morbidities Using Models of Partial-body Irradiation with Marginal Bone Marrow Sparing and Whole Thorax Lung Irradiation
- PMID: 33009295
- PMCID: PMC9440605
- DOI: 10.1097/HP.0000000000001346
Acute Radiation-induced Lung Injury in the Non-human Primate: A Review and Comparison of Mortality and Co-morbidities Using Models of Partial-body Irradiation with Marginal Bone Marrow Sparing and Whole Thorax Lung Irradiation
Abstract
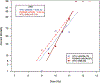
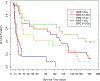
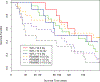
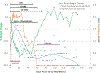
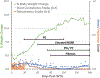
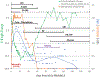
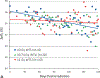
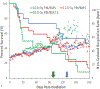
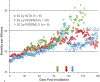
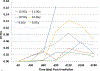
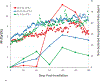
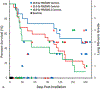
The nonhuman primate, rhesus macaque, is a relevant animal model that has been used to determine the efficacy of medical countermeasures to mitigate major signs of morbidity and mortality of radiation-induced lung injury. Herein, a literature review of published studies showing the evolution of lethal lung injury characteristic of the delayed effects of acute radiation exposure between the two significantly different exposure protocols, whole thorax lung irradiation and partial-body irradiation with bone marrow sparing in the nonhuman primate, is provided. The selection of published data was made from the open literature. The primary studies conducted at two research sites benefitted from the similarity of major variables; namely, both sites used rhesus macaques of approximate age and body weight and radiation exposure by LINAC-derived 6 MV photons at dose rates of 0.80 Gy min and 1.00 Gy min delivered to the midline tissue via bilateral, anterior/posterior, posterior/anterior geometry. An advantage relative to sex difference resulted from the use of male and female macaques by the Maryland and the Washington sites, respectively. Subject-based medical management was used for all macaques. The primary studies (6) provided adequate data to establish dose response relationships within 180 d for the radiation-induced lung injury consequent to whole thorax lung irradiation (male vs. female) and partial-body irradiation with bone marrow sparing exposure protocols (male). The dose response relationships established by probit analyses vs. linear dose relationships were characterized by two main parameters or dependent variables, a slope and LD50/180. Respective LD50/180 values for the primary studies that used whole thorax lung irradiation for respective male and female nonhuman primates were 10.24 Gy [9.87, 10.52] (n = 76, male) and 10.28 Gy [9.68, 10.92] (n = 40, female) at two different research sites. The respective slopes were steep at 1.73 [0.841, 2.604] and 1.15 [0.65, 1.65] probits per linear dose. The LD50/180 value and slope derived from the dose response relationships for the partial-body irradiation with bone marrow sparing exposure was 9.94 Gy [9.35, 10.29] (n = 87) and 1.21 [0.70, 1.73] probits per linear dose. A secondary study (1) provided data on limited control cohort of nonhuman primates exposed to whole thorax lung irradiation. The data supported the incidence of clinical, radiographic, and histological indices of the dose-dependent lung injury in the nonhuman primates. Tertiary studies (6) provided data derived from collaboration with the noted primary and secondary studies on control cohorts of nonhuman primates exposed to whole thorax lung irradiation and partial-body irradiation with bone marrow sparing exposure. These studies provided a summary of histological evidence of fibrosis, inflammation and reactive/proliferative changes in pneumonocytes characteristic of lung injury and data on biomarkers for radiation-induced lung injury based on matrix-assisted laser desorption ionization-mass spectrometry imaging and gene expression approaches. The available database in young rhesus macaques exposed to whole thorax lung irradiation or partial-body irradiation with bone marrow sparing using 6 MV LINAC-derived radiation with medical management showed that the dose response relationships were equivalent relative to the primary endpoint all-cause mortality. Additionally, the latency, incidence, severity, and progression of the clinical, radiographic, and histological indices of lung injury were comparable. However, the differences between the exposure protocols are remarkable relative to the demonstrated time course between the multiple organ injury of the acute radiation syndrome and that of the delayed effects of acute radiation exposure, respectively.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Asano S. Multi-organ involvement: lessons from the experience of one victim of the Tokai-mura criticality accident. Br J Radiol 78; 2005.
-
- Baranov AE, Selidovkin GD, Butturini A, Gale RP. Hematopoietic recovery after 10-Gy acute total body radiation. Blood 83: 596–599; 1994. - PubMed
-
- Berkely FJ. Managing the adverse effects of radiation therapy. AM Fam Physician 82: 381–388; 2010. - PubMed
-
- Boittin F-X, Martigne P, Mayol J-F, Denis J, Raffin F, Coulon D, Grenier N, Drouet M, Herodin F. Experimental quantification of delayed radiation-indduced organ damage in highly irradiated rats with bone marrow protection: effect of radiation dose and organ sensitivity. Health Phys 109: 134–144; 2015. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

