A petascale automated imaging pipeline for mapping neuronal circuits with high-throughput transmission electron microscopy
- PMID: 33009388
- PMCID: PMC7532165
- DOI: 10.1038/s41467-020-18659-3
A petascale automated imaging pipeline for mapping neuronal circuits with high-throughput transmission electron microscopy
Abstract
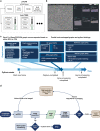
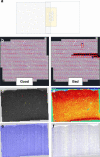
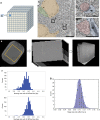
Electron microscopy (EM) is widely used for studying cellular structure and network connectivity in the brain. We have built a parallel imaging pipeline using transmission electron microscopes that scales this technology, implements 24/7 continuous autonomous imaging, and enables the acquisition of petascale datasets. The suitability of this architecture for large-scale imaging was demonstrated by acquiring a volume of more than 1 mm3 of mouse neocortex, spanning four different visual areas at synaptic resolution, in less than 6 months. Over 26,500 ultrathin tissue sections from the same block were imaged, yielding a dataset of more than 2 petabytes. The combined burst acquisition rate of the pipeline is 3 Gpixel per sec and the net rate is 600 Mpixel per sec with six microscopes running in parallel. This work demonstrates the feasibility of acquiring EM datasets at the scale of cortical microcircuits in multiple brain regions and species.
Conflict of interest statement
Harvard University has filed patent applications regarding GridTape (WO2017184621A1) and the prototype reel-to-reel TEM imaging stage (WO2018089578A1) on behalf of the investigators (B.J.G., W-C.A.L.) and others. C.S.O. and M.F.M. have a financial interest in Voxa. The remaining authors declare no competing interests.
Figures


References
-
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode caenorhabditis elegans. Philos. Trans. R. Soc. B Biol. Sci. 1986;314:1–340. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

