Dupilumab is effective in type 2-high asthma patients receiving high-dose inhaled corticosteroids at baseline
- PMID: 33010038
- PMCID: PMC7820970
- DOI: 10.1111/all.14611
Dupilumab is effective in type 2-high asthma patients receiving high-dose inhaled corticosteroids at baseline
Abstract
Background: Dupilumab blocks the shared receptor component for interleukin (IL)-4/IL-13, key drivers of type 2 inflammation. In phase 2b (NCT01854047) and phase 3 LIBERTY ASTHMA QUEST (NCT02414854), add-on dupilumab 200/300 mg every 2 weeks (q2w) reduced severe exacerbations, improved prebronchodilator (pre-BD) forced expiratory volume in 1 second (FEV1 ) and quality of life measures, and it was generally well tolerated in patients with uncontrolled, persistent (phase 2b), or moderate-to-severe (phase 3) asthma.
Methods: In patients on high-dose inhaled corticosteroids (ICS) with type 2-high asthma (subgroups including baseline blood eosinophils ≥150/300 cells/µL and/or fractional exhaled nitric oxide [FeNO] ≥25 ppb), annualized severe exacerbation rates over the treatment period, changes from baseline in pre-BD FEV1 and asthma control (5-item asthma control questionnaire [ACQ-5]) were analyzed.
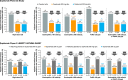
Results: In high-dose ICS type 2-high subgroups, dupilumab 200/300 mg q2w vs placebo in the phase 2b (24 weeks) and phase 3 (52 weeks) studies significantly reduced severe exacerbations by 55%-69%/57%-60% (all P<.05) and 53%-69%/48%-66% (all P < .001), respectively, except in patients with ≥ 300 eosinophils/µL in phase 2b study (24%/50% (P = .52/0.15). Across subgroups, pre-BD FEV1 improved by 0.18-0.22 L/0.19-0.24 L (all P < .05) and 0.23-0.36 L/0.15-0.25 L (all P < .01) and ACQ-5 scores were reduced by 0.46-0.55/0.47-0.85 (all P < .05) and 0.38-0.50/0.24-0.30 (all P < .05), respectively, except dupilumab 200 mg q2w in phase 2b in patients with FeNO ≥ 25 ppb (0.41; P = .09). Dupilumab was also effective in patients taking medium-dose ICS.
Conclusion: Dupilumab significantly reduced severe exacerbations and improved lung function and asthma control in patients with type 2-high asthma on high-dose ICS at baseline.
Keywords: asthma control; exacerbations; inhaled corticosteroids; moderate-to-severe asthma; prebronchodilator FEV1.
© 2020 The Authors. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
Arnaud Bourdin reports non‐financial support from GlaxoSmithKline (GSK), during the conduct of the study; personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, Chiesi, GSK, Novartis, and Sanofi‐Regeneron; other from Acceleron, Actelion, Galapagos, MSD, Nuvaira, Pulmonx, United Therapeutic, and Vertex, outside the submitted work. Alberto A. Papi reports grants, personal fees, non‐financial support and other from GlaxoSmithKline, Boehringer Ingelheim, Chiesi Farmaceutici, and TEVA; grants, personal fees and non‐financial support from AstraZeneca and Menarini; personal fees, non‐financial support and other from Mundipharma, Zambon, Novartis, and Sanofi/Regeneron; personal fees from Roche and Edmondpharma; and grants from Fondazione Maugeri and Fondazione Chiesi; outside the submitted work. Jonathan Corren reports research support from Sanofi outside the submitted work. J. Christian Virchow reports personal fees from AstraZeneca, Avontec, Bayer, Bencard, Bionorica, Boehringer Ingelheim, Chiesi, Essex/Schering‐Plough, GSK, Janssen‐Cilag, Leti, MEDA, Merck, MSD, Mundipharma, Novartis, Nycomed/Altana, Pfizer, Revotar, Sandoz‐Hexal, Stallergens, Teva, UCB/Schwarz‐Pharma, Zydus/Cadila, and possibly others; other for Avontec, Boehringer Ingelheim, Chiesi, Essex/Schering‐Plough, GSK, Janssen‐Cilag, MEDA, MSD, Mundipharma, Novartis, Regeneron, Revotar, Roche, Sanofi‐Aventis, Sandoz‐Hexal, Teva, UCB/Schwarz‐Pharma, and possibly others; and research grants from Deutsche Forschungsgesellschaft, Land Mecklenburg‐Vorpommern, GSK, and MSD. Megan S. Rice reports personal fees and other from Sanofi, outside the submitted work. Yamo Deniz reports personal fees and other from Regeneron Pharmaceuticals, Inc, outside the submitted work. Michel Djandji reports personal fees and other from Sanofi, outside the submitted work. Paul Rowe reports personal fees and other from Sanofi, outside the submitted work. Ian D. Pavord reports personal fees from AstraZeneca, Boehringer Ingelheim, Aerocrine, Almirall, Novartis, GlaxoSmithKline, Genentech, and Regeneron; other from Teva, Chiesi, Sanofi, Circassia, and Knopp; grants from NIHR, outside the submitted work.
Figures


References
-
- Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF‐κB activity through induction of IκB synthesis. Science. 1995;270(5234):286‐290. - PubMed
-
- Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102(4 Pt 2):S17‐S22. - PubMed
-
- Beasley R, Harper J, Bird G, Maijers I, Weatherall M, Pavord ID. Inhaled corticosteroid therapy in adult asthma. Time for a new therapeutic dose terminology. Am J Respir Crit Care Med. 2019;199(12):1471‐1477. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

