Synthesis of Unprecedented 4d/4f-Polypnictogens
- PMID: 33010187
- PMCID: PMC7986065
- DOI: 10.1002/chem.202003905
Synthesis of Unprecedented 4d/4f-Polypnictogens
Abstract
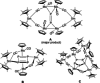
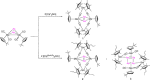
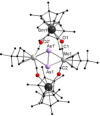
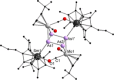
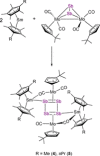
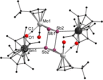
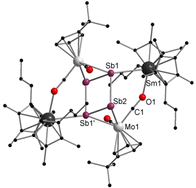
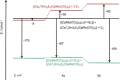
A series of 4d/4f-polyarsenides, -polyarsines and -polystibines was obtained by reduction of the Mo-pnictide precursor complexes [{Cpt Mo(CO)2 }2 (μ,η2:2 -E2 )] (E=As, Sb; Cpt =tBu substituted cyclopentadienyl) with two different divalent samarocenes [Cp*2 Sm] and [(CpMe4nPr )2 Sm]. For the reductive conversion of the Mo-stibide only one product was isolated, featuring a planar tetrastibacyclobutadiene moiety as an unprecedented ligand for organometallic compounds. For the corresponding Mo-arsenide a tetraarsacyclobutadiene and a second species with a side-on coordinated As2 2- anion was isolated. The latter can be considered as reaction intermediate for the formation of the tetraarsacyclobutadiene.
Keywords: lanthanides; molybdenum; polyarsenides; polystibides; reduction.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Faessler T. F., Structure and Bonding, Vol. 140 (Ed.: Mingos M. P.), Springer Verlag, Berlin, 2011.
-
- Zide J. M. O., Kleiman-Shwarsctein A., Strandwitz N. C., Zimmerman J. D., Steenblock-Smith T., Gossard A. C., Forman A., Ivanovskaya A., Stucky G. D., Appl. Phys. Lett. 2006, 88, 162103.
-
- None
-
- Detzel M., Mohr T., Scherer O. J., Wolmershäuser G., Angew. Chem. Int. Ed. Engl. 1994, 33, 1110–1112;
- Angew. Chem. 1994, 106, 1142–1144;
-
- Scherer O. J., Winter R., Wolmershäuser G., Z. Anorg. Allg. Chem. 1993, 619, 827–835.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

