Liquid Phase Separation Controlled by pH
- PMID: 33010236
- PMCID: PMC7642337
- DOI: 10.1016/j.bpj.2020.07.044
Liquid Phase Separation Controlled by pH
Abstract
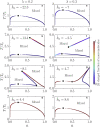
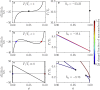
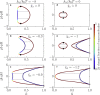
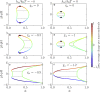
We present a minimal model to study the effects of pH on liquid phase separation of macromolecules. Our model describes a mixture composed of water and macromolecules that exist in three different charge states and have a tendency to phase separate. This phase separation is affected by pH via a set of chemical reactions describing protonation and deprotonation of macromolecules, as well as self-ionization of water. We consider the simple case in which interactions are captured by Flory-Huggins interaction parameters corresponding to Debye screening lengths shorter than a nanometer, which is relevant to proteins inside biological cells under physiological conditions. We identify the conjugate thermodynamic variables at chemical equilibrium and discuss the effective free energy at fixed pH. First, we study phase diagrams as a function of macromolecule concentration and temperature at the isoelectric point of the macromolecules. We find a rich variety of phase diagram topologies, including multiple critical points, triple points, and first-order transition points. Second, we change the pH relative to the isoelectric point of the macromolecules and study how phase diagrams depend on pH. We find that these phase diagrams as a function of pH strongly depend on whether oppositely charged macromolecules or neutral macromolecules have a stronger tendency to phase separate. One key finding is that we predict the existence of a reentrant behavior as a function of pH. In addition, our model predicts that the region of phase separation is typically broader at the isoelectric point. This model could account for both in vitro phase separation of proteins as a function of pH and protein phase separation in yeast cells for pH values close to the isoelectric point of many cytosolic proteins.
Copyright © 2020 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Alberts B. Garland Science; New York: 2017. Molecular Biology of the Cell.
-
- Brangwynne C.P., Eckmann C.R., Hyman A.A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

