Association Between Time to Colonoscopy After Positive Fecal Testing and Colorectal Cancer Outcomes: A Systematic Review
- PMID: 33010414
- PMCID: PMC7527352
- DOI: 10.1016/j.cgh.2020.09.048
Association Between Time to Colonoscopy After Positive Fecal Testing and Colorectal Cancer Outcomes: A Systematic Review
Abstract
Background & aims: Colonoscopy is required following a positive fecal screening test for colorectal cancer (CRC). It remains unclear to what extent time to colonoscopy is associated with CRC-related outcomes. We performed a systematic review to elucidate this relationship.
Methods: An electronic search was performed through April 2020 for studies reporting associations between time from positive fecal testing to colonoscopy and outcomes including CRC incidence (primary outcome), CRC stage at diagnosis, and/or CRC-specific mortality. Our primary objective was to quantify these relationships following positive fecal immunochemical testing (FIT). Two authors independently performed screening, abstraction, and risk of bias assessments.
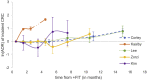
Results: From 1,612 initial studies, 8 were included in the systematic review, with 5 reporting outcomes for FIT. Although meta-analysis was not possible, consistent trends between longer time delays and worse outcomes were apparent in all studies. Colonoscopy performed beyond 9 months from positive FIT compared to within 1 month was significantly associated with a higher incidence of CRC, with adjusted odds ratios (AORs) of 1.75 and 1.48 in the two largest studies. These studies also reported significant associations between colonoscopy performed beyond 9 months and higher incidence of advanced stage CRC (stage III or IV) at diagnosis, with AORs of 2.79 and 1.55, respectively.
Conclusions: Colonoscopy for positive FIT should not be delayed beyond 9 months. Given the additional time required for urgent referrals and surgical planning for CRC, colonoscopy should ideally be performed well in advance of 9 months following a positive FIT.
Keywords: Colonoscopy; Colorectal Neoplasms; Mass Screening.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




Comment in
-
Patienten mit positivem iFOBT müssen rasch zur Koloskopie.MMW Fortschr Med. 2022 Apr;164(Suppl 1):16-17. doi: 10.1007/s15006-022-0726-1. MMW Fortschr Med. 2022. PMID: 35359285 Free PMC article. German. No abstract available.
References
-
- GBD 2017 Colorectal Cancer Collaborators The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:913–933. - PMC - PubMed
-
- Robertson D.J., Lee J.K., Boland C.R. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: a Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;152:1217–1237.e3. - PubMed
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

