The novel coronavirus Disease-2019 (COVID-19): Mechanism of action, detection and recent therapeutic strategies
- PMID: 33010669
- PMCID: PMC7513802
- DOI: 10.1016/j.virol.2020.08.011
The novel coronavirus Disease-2019 (COVID-19): Mechanism of action, detection and recent therapeutic strategies
Abstract
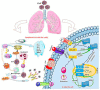
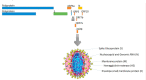
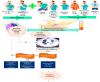
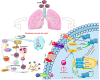
Novel coronavirus SARS-CoV-2, designated as COVID-19 by the World Health Organization (WHO) on the February 11, 2020, is one of the highly pathogenic β-coronaviruses which infects human. Early diagnosis of COVID-19 is the most critical step to treat infection. The diagnostic tools are generally molecular methods, serology and viral culture. Recently CRISPR-based method has been investigated to diagnose and treat coronavirus infection. The emergence of 2019-nCoV during the influenza season, has led to the extensive use of antibiotics and neuraminidase enzyme inhibitors, taken orally and intravenously. Currently, antiviral inhibitors of SARS and MERS spike proteins, neuraminidase inhibitors, anti-inflammatory drugs and EK1 peptide are the available therapeutic options for SARS-CoV-2 infected individuals. In addition, Chloroquine, which was previously used for malarial and autoimmune disease, has shown efficacy in the 2019-nCoV infection treatment. In severe hypoxaemia, a combination of antibiotics, α-interferon, lopinavir and mechanical ventilation can effectively mitigate the symptoms. Comprehensive knowledge on the innate and adaptive immune responses, will make it possible to propose potent antiviral drugs with their effective therapeutic measures for the prevention of viral infection. This therapeutic strategy will help patients worldwide to protect themselves against severe and fatal viral infections, that potentially can evolve and develop drug resistance, and to reduce mortality rates.
Keywords: Coronavirus; Immune response; Pathogen; Respiratory syndrome; Treatment.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
The authors declared that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

