The Origin of Ovarian Cancer Species and Precancerous Landscape
- PMID: 33011111
- PMCID: PMC7786078
- DOI: 10.1016/j.ajpath.2020.09.006
The Origin of Ovarian Cancer Species and Precancerous Landscape
Abstract
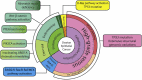
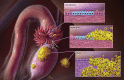
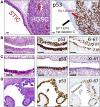
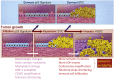
Unlike other human cancers, in which all primary tumors arise de novo, ovarian epithelial cancers are primarily imported from either endometrial or fallopian tube epithelium. The prevailing paradigm in the genesis of high-grade serous carcinoma (HGSC), the most common ovarian cancer, posits to its development in fallopian tubes through stepwise tumor progression. Recent progress has been made not only in gathering terabytes of omics data but also in detailing the histologic-molecular correlations required for looking into, and making sense of, the tissue origin of HGSC. This emerging paradigm is changing many facets of ovarian cancer research and routine gynecology practice. The precancerous landscape in fallopian tubes contains multiple concurrent precursor lesions, including serous tubal intraepithelial carcinoma (STIC), with genetic heterogeneity providing a platform for HGSC evolution. Mathematical models imply that a prolonged time (decades) elapses from the development of a TP53 mutation, the earliest known molecular alteration, to an STIC, followed by a shorter span (6 years) for progression to an HGSC. Genetic predisposition accelerates the trajectory. This timeline may allow for the early diagnosis of HGSC and STIC, followed by intent-to-cure surgery. This review discusses the recent advances in this tubal paradigm and its biological and clinical implications, alongside the promise and challenge of studying STIC and other precancerous lesions of HGSC.
Copyright © 2021 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Kuhn T.S., Hacking I. ed 4. The University of Chicago Press; Chicago: 2012. The Structure of Scientific Revolutions.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

