Vancomycin Hypersensitivity Reactions Documented in Electronic Health Records
- PMID: 33011300
- PMCID: PMC7870516
- DOI: 10.1016/j.jaip.2020.09.027
Vancomycin Hypersensitivity Reactions Documented in Electronic Health Records
Abstract
Background: Vancomycin, the most common antimicrobial used in US hospitals, can cause diverse adverse reactions, including hypersensitivity reactions (HSRs). Yet, little is known about vancomycin reactions documented in electronic health records.
Objective: To describe vancomycin HSR epidemiology from electronic health record allergy data.
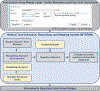
Methods: This was a cross-sectional study of patients with 1 or more encounter from 2017 to 2019 and an electronic health record vancomycin drug allergy label (DAL) in 2 US health care systems. We determined prevalence and trends of vancomycin DALs and assessed active DALs by HSR phenotype determined from structured (coded) and unstructured (free-text) data using natural language processing. We investigated demographic associations with documentation of vancomycin red man syndrome (RMS).
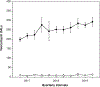
Results: Among 4,490,618 patients, 14,426 (0.3%) had a vancomycin DAL with 18,761 documented reactions (2,248 [12.0%] free-text). Quarterly mean vancomycin DALs added were 253 ± 12 and deleted were 12 ± 2. Of 18,761 vancomycin HSRs, 7,903 (42.1%) were immediate phenotypes and 3,881 (20.7%) were delayed phenotypes. Common HSRs were rash (32% of HSRs) and RMS (16% of HSRs). Anaphylaxis was coded in 6% cases of HSRs. Drug reaction eosinophilia and systemic symptoms syndrome was the most common coded vancomycin severe cutaneous adverse reaction. RMS documentation was more likely for males (odds ratio, 1.30; 95% CI, 1.17-1.44) and less likely for blacks (odds ratio, 0.59; 95% CI, 0.47-0.75).
Conclusions: Vancomycin causes diverse adverse reactions, including common (eg, RMS) and severe (eg, drug reaction eosinophilia and systemic symptoms syndrome) reactions entered as DAL free-text. Anaphylaxis comprised 6% of documented vancomycin HSRs, although true vancomycin IgE-mediated reactions are exceedingly rare. Improving vancomycin DAL documentation requires more coded entry options, including a coded entry for RMS.
Keywords: Allergy; Drug allergy label; Drug reaction eosinophilia and systemic symptoms syndrome; Electronic health record; Epidemiology; Hypersensitivity; Infusion reaction; Phenotype; Red man syndrome; Vancomycin.
Copyright © 2020 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Vancomycin Hypersensitivity: It Is Not Always What It Seems.J Allergy Clin Immunol Pract. 2021 Feb;9(2):913-915. doi: 10.1016/j.jaip.2020.10.040. J Allergy Clin Immunol Pract. 2021. PMID: 33551043 No abstract available.
References
-
- Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666–74. - PubMed
-
- Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–55. - PubMed
-
- Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352(14):1436–44. - PubMed
-
- Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr., Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98. - PubMed
-
- Stevens VW, Nelson RE, Schwab-Daugherty EM, Khader K, Jones MM, Brown KA, et al. Comparative Effectiveness of Vancomycin and Metronidazole for the Prevention of Recurrence and Death in Patients With Clostridium difficile Infection. JAMA Intern Med. 2017;177(4):546–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

