β-catenin regulates muscle glucose transport via actin remodelling and M-cadherin binding
- PMID: 33011305
- PMCID: PMC7568189
- DOI: 10.1016/j.molmet.2020.101091
β-catenin regulates muscle glucose transport via actin remodelling and M-cadherin binding
Abstract
Objective: Skeletal muscle glucose disposal following a meal is mediated through insulin-stimulated movement of the GLUT4-containing vesicles to the cell surface. The highly conserved scaffold-protein β-catenin is an emerging regulator of vesicle trafficking in other tissues. Here, we investigated the involvement of β-catenin in skeletal muscle insulin-stimulated glucose transport.
Methods: Glucose homeostasis and transport was investigated in inducible muscle specific β-catenin knockout (BCAT-mKO) mice. The effect of β-catenin deletion and mutation of β-catenin serine 552 on signal transduction, glucose uptake and protein-protein interactions were determined in L6-G4-myc cells, and β-catenin insulin-responsive binding partners were identified via immunoprecipitation coupled to label-free proteomics.
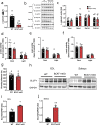
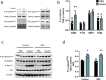
Results: Skeletal muscle specific deletion of β-catenin impaired whole-body insulin sensitivity and insulin-stimulated glucose uptake into muscle independent of canonical Wnt signalling. In response to insulin, β-catenin was phosphorylated at serine 552 in an Akt-dependent manner, and in L6-G4-myc cells, mutation of β-cateninS552 impaired insulin-induced actin-polymerisation, resulting in attenuated insulin-induced glucose transport and GLUT4 translocation. β-catenin was found to interact with M-cadherin in an insulin-dependent β-cateninS552-phosphorylation dependent manner, and loss of M-cadherin in L6-G4-myc cells attenuated insulin-induced actin-polymerisation and glucose transport.
Conclusions: Our data suggest that β-catenin is a novel mediator of glucose transport in skeletal muscle and may contribute to insulin-induced actin-cytoskeleton remodelling to support GLUT4 translocation.
Keywords: Actin-remodelling; Beta-catenin; GLUT4 trafficking; Glucose transport; M-cadherin.
Copyright © 2020 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures






References
-
- DeFronzo R.A., Ferrannini E. Regulation of hepatic glucose metabolism in humans. Diabetes/Metabolism Research and Reviews. 1987;3(2):415–459. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

