Novel acyl carbamates and acyl / diacyl ureas show in vitro efficacy against Toxoplasma gondii and Cryptosporidium parvum
- PMID: 33011650
- PMCID: PMC7529613
- DOI: 10.1016/j.ijpddr.2020.08.006
Novel acyl carbamates and acyl / diacyl ureas show in vitro efficacy against Toxoplasma gondii and Cryptosporidium parvum
Abstract
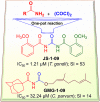
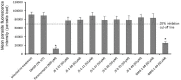
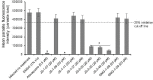
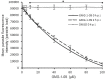
Toxoplasma gondii and Cryptosporidium parvum are protozoan parasites that are highly prevalent and opportunistically infect humans worldwide, but for which completely effective and safe medications are lacking. Herein, we synthesized a series of novel small molecules bearing the diacyl urea scaffold and related structures, and screened them for in vitro cytotoxicity and antiparasitic activity against T. gondii and C. parvum. We identified one compound (GMG-1-09), and four compounds (JS-1-09, JS-2-20, JS-2-35 and JS-2-49) with efficacy against C. parvum and T. gondii, respectively, at low micromolar concentrations and showed appreciable selectivity in human host cells. Among the four compounds with efficacy against T. gondii, JS-1-09 representing the diacyl urea scaffold was the most effective, with an anti-Toxoplasma IC50 concentration (1.21 μM) that was nearly 53-fold lower than its cytotoxicity IC50 concentration, indicating that this compound has a good selectivity index. The other three compounds (JS-2-20, JS-2-35 and JS-2-49) were structurally more divergent from JS-1-09 as they represent the acyl urea and acyl carbamate scaffold. This appeared to correlate with their anti-Toxoplasma activity, suggesting that these compounds' potency can likely be enhanced by selective structural modifications. One compound, GMG-1-09 representing acyl carbamate scaffold, depicted in vitro efficacy against C. parvum with an IC50 concentration (32.24 μM) that was 14-fold lower than its cytotoxicity IC50 concentration in a human intestinal cell line. Together, our studies unveil a series of novel synthetic acyl/diacyl urea and acyl carbamate scaffold-based small molecule compounds with micromolar activity against T. gondii and C. parvum that can be explored further for the development of the much-needed novel anti-protozoal drugs.
Keywords: Acyl carbamate; Acyl urea; Cryptosporidium parvum; Diacyl urea; Drug discovery; Toxoplasma gondii.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Abrahamsen M.S., Templeton T.J., Enomoto S., Abrahante J.E., Zhu G., Lancto C.A., Deng M., Liu C., Widmer G., Tzipori S., Buck G.A., Xu P., Bankier A.T., Dear P.H., Konfortov B.A., Spriggs H.F., Iyer L., Anantharaman V., Aravind L., Kapur V. Complete genome sequence of apicomplexan. C. parvum. Science. 2004;304:441–445. doi: 10.1126/science.1094786. - DOI - PubMed
-
- Arrowood M.J., Sterling C.R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J. Parasitol. 1987;73:314–319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

