Two-year outcomes of the MINIject drainage system for uncontrolled glaucoma from the STAR-I first-in-human trial
- PMID: 33011690
- PMCID: PMC8685654
- DOI: 10.1136/bjophthalmol-2020-316888
Two-year outcomes of the MINIject drainage system for uncontrolled glaucoma from the STAR-I first-in-human trial
Abstract
Background/aims: The current study evaluates the efficacy and safety of the stand-alone implantation of the MINIject (iSTAR Medical, Wavre, Belgium) supraciliary, microinvasive glaucoma drainage device in patients with medically uncontrolled open-angle glaucoma.
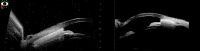
Methods: This prospective, multicentre, first-in-human, single-arm interventional study evaluated stand-alone, ab interno implantation in 25 patients of a 5 mm long uveoscleral device made of STAR biocompatible material, which is a soft, microporous, flexible silicone. The primary outcome was the reduction of intraocular pressure (IOP) at 6 months compared with baseline, and follow-up continued until 2 years for 21 patients. Secondary outcomes included success defined as diurnal IOP of ≤21 mmHg and >5 mmHg with an IOP reduction of 20% without (complete) or with/without (qualified) glaucoma medication.
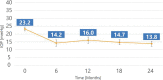
Results: Mean baseline IOP was 23.2±2.9 mmHg on 2.0±1.1 glaucoma medication ingredients and decreased to 13.8±3.5 mmHg (-40.7% reduction) on 1.0±1.3 medications 2 years after implantation. Complete success was achieved in 47.6% of patients (10/21) and qualified success in 100% of patients (21/21) at the 2-year follow-up. All patients achieved a 20% IOP reduction with 48% of patients medication-free. No serious ocular adverse events or additional glaucoma surgery were reported. Mean central endothelial cell density (ECD) mildly decreased from 2411 cells/mm2 (n=26) to 2341 cells/mm2 (n=21) at 24 months, which represents a 5% decrease for matched eyes. No patient had a ≥30% decrease in central ECD.
Conclusion: This first-in-human study on the stand-alone implantation of the MINIject supraciliary drainage system shows promising IOP-lowering results and medication reduction over 24 months with few adverse events.
Trial registration number: NCT03193736.
Keywords: Anterior chamber; Aqueous humour; Glaucoma; Intraocular pressure; Treatment surgery.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PD: iSTAR Medical, Alcon, Allergan, Glaukos, Santen, Thea; CH: iSTAR Medical, Thea Pharma; GMD: Alcon, Allergan, Bausch & Lomb, Glaukos, Labtician, Novartis, MST, Santen, Sight Sciences; KPR, AK, EC: no financial interests; ZH: Vice President of Regulatory and Clinical at iSTAR Medical; IKA: Aequus, Aerie Pharmaceuticals, Alcon, Allergan, ArcScan, Bausch & Lomb, Beaver Visitec, Camras Vision, Carl Zeiss Meditec, CorNeat Vision, Ellex, ElutiMed, Equinox, Genentech, Glaukos, Gore, Iantech, InjectSense, Iridex, iSTAR Medical, Ivantis, Johnson & Johnson Vision, KeLoTec, LayerBio, Leica Microsystems, MicroOptx, New World Medical, Omega Ophthalmics, PolyActiva, Sanoculis, Santen, Science Based Health, Sight Sciences, Stroma, TrueVision, Vizzari.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
