Inhaled corticosteroids as treatment for adolescent asthma: effects on adult anxiety-related outcomes in a murine model
- PMID: 33011818
- PMCID: PMC8787845
- DOI: 10.1007/s00213-020-05666-x
Inhaled corticosteroids as treatment for adolescent asthma: effects on adult anxiety-related outcomes in a murine model
Erratum in
-
Correction to: Inhaled corticosteroids as treatment for adolescent asthma: effects on adult anxiety-related outcomes in a murine model.Psychopharmacology (Berl). 2021 Apr;238(4):1225. doi: 10.1007/s00213-021-05778-y. Psychopharmacology (Berl). 2021. PMID: 33566114 No abstract available.
Abstract
Rationale: Allergic asthma, typically controlled with inhaled corticosteroids (ICS), is the leading chronic health condition for youth under 18 years of age. During this peri-adolescent period, significant brain maturation occurs. Prior studies indicate that both chronic inflammation and corticosteroid medications increase risk for developing an internalizing disorder like anxiety.
Objectives: To determine if chronic ICS treatments exacerbate or alleviate anxiety symptoms associated with developmental allergic asthma, we used a mouse model to isolate the influence of ICS (fluticasone propionate, FLU) vs. airway inflammation (induced with house dust mite extract, HDM).
Methods: During development, male and female BALB/cJ mice were repeatedly exposed to HDM or saline plus one of four FLU doses (none/vehicle, low, moderate, or high). In adulthood, we assessed lung inflammation, circulating and excreted corticosteroids, anxiety-like behavior, and gene expression in stress and emotion regulation brain regions.
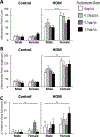
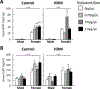
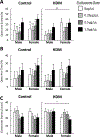
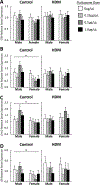
Results: FLU treatment decreased body weight and anxiety-like behavior and increased fecal corticosterone metabolite concentrations and Crhr2 gene expression in ventral hippocampus. FLU effects were only observed in saline/non-HDM-exposed mice, and the FLU doses used did not significantly decrease HDM-induced airway inflammation. Females had greater serum and fecal corticosterone concentrations, less anxiety-like behavior, and lower Crhr1 gene expression in ventral hippocampus and prefrontal cortex than males.
Conclusions: These findings suggest that steroid medications for youth with allergic asthma may not exacerbate anxiety-related symptoms, and that they should be avoided in children/adolescents without a health condition. The results are informative to future work on the use of corticosteroid medications during childhood or adolescent development.
Keywords: Adolescence; Anxiety; Asthma; Comorbidity; Development; HPA axis; Inhaled corticosteroids.
Conflict of interest statement
The authors have no competing interests to declare.
Figures


References
-
- Akinbami LJ, Moorman JE, Bailey C, et al. (2012) Trends in Asthm a Prevalence, Health Care Use, and Mortality in the United States, 2001–2010. NCHS Data Brief 94:1–8 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

