Retinal biomarkers for Alzheimer's disease and vascular cognitive impairment and dementia (VCID): implication for early diagnosis and prognosis
- PMID: 33011937
- PMCID: PMC7732888
- DOI: 10.1007/s11357-020-00252-7
Retinal biomarkers for Alzheimer's disease and vascular cognitive impairment and dementia (VCID): implication for early diagnosis and prognosis
Abstract
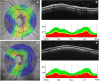
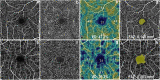
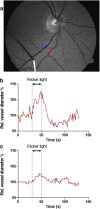
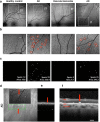
Cognitive impairment and dementia are major medical, social, and economic public health issues worldwide with significant implications for life quality in older adults. The leading causes are Alzheimer's disease (AD) and vascular cognitive impairment/dementia (VCID). In both conditions, pathological alterations of the cerebral microcirculation play a critical pathogenic role. Currently, the main pathological biomarkers of AD-β-amyloid peptide and hyperphosphorylated tau proteins-are detected either through cerebrospinal fluid (CSF) or PET examination. Nevertheless, given that they are invasive and expensive procedures, their availability is limited. Being part of the central nervous system, the retina offers a unique and easy method to study both neurodegenerative disorders and cerebral small vessel diseases in vivo. Over the past few decades, a number of novel approaches in retinal imaging have been developed that may allow physicians and researchers to gain insights into the genesis and progression of cerebromicrovascular pathologies. Optical coherence tomography (OCT), OCT angiography, fundus photography, and dynamic vessel analyzer (DVA) are new imaging methods providing quantitative assessment of retinal structural and vascular indicators-such as thickness of the inner retinal layers, retinal vessel density, foveal avascular zone area, tortuosity and fractal dimension of retinal vessels, and microvascular dysfunction-for cognitive impairment and dementia. Should further studies need to be conducted, these retinal alterations may prove to be useful biomarkers for screening and monitoring dementia progression in clinical routine. In this review, we seek to highlight recent findings and current knowledge regarding the application of retinal biomarkers in dementia assessment.
Keywords: Alzheimer’s disease; Dementia; OCT angiography; Retinal biomarkers; Retinal imaging.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
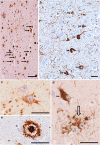
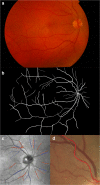
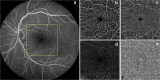
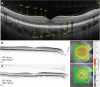
References
-
- 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020. - PubMed
-
- Liesz A. The vascular side of Alzheimer’s disease. Science. 2019;365:223–224. - PubMed
-
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert MO, Jicha GA, Nordberg A, Pasquier F, Rabinovici G, Robert P, Rowe C, Salloway S, Sarazin M, Epelbaum S, de Souza LC, Vellas B, Visser PJ, Schneider L, Stern Y, Scheltens P, Cummings JL. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. - PubMed
-
- Budson AE, Solomon PR. New diagnostic criteria for Alzheimer’s disease and mild cognitive impairment for the practical neurologist. Pract Neurol. 2012;12:88–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

