Bayesian analysis of the epidemiology of bleeding in critically ill children
- PMID: 33012582
- PMCID: PMC8005501
- DOI: 10.1016/j.jcrc.2020.09.028
Bayesian analysis of the epidemiology of bleeding in critically ill children
Abstract
Purpose: We updated our findings on the epidemiology of clinically relevant bleeding (CRB) in critically ill children. We also determined the concordance of CRB as defined by the International Society of Thrombosis and Haemostasis, i.e., ISTH definition, and characteristics identified by pediatric intensivists in a recent survey, i.e., survey definition.
Methods: In a prospective cohort study, we included children <18 years old who were admitted to the pediatric intensive care unit for >1 day. We followed them daily for bleeding. Bayesian inference was used as the primary analytic tool to incorporate our prior findings.
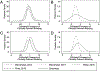
Results: Using the ISTH definition, the estimated frequency of CRB was 10.0% (95% credible interval, CrI: 7.6%, 12.8%) from 41 of 405 children who had CRB. The estimated frequency from 4 of 12 adolescents >13 years old who received mechanical ventilation or vasopressor support and had CRB was 32.9% (95% CrI: 12.0%, 58.8%). Using the survey definition, the estimated frequency of CRB for the entire cohort was 10.8% (95% CrI: 8.3%, 13.8%). Concordance between definitions for each bleeding event was 0.40 (95% confidence interval: 0.27, 0.52).
Conclusions: Our updated findings highlight the high frequency of CRB regardless of definition used for CRB.
Keywords: Adolescent; Intensive care unit; Mechanical ventilation; Risk factor; Transfusion.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None of the authors declare any conflicts of interest.
Figures

References
-
- White LJ, Fredericks R, Mannarino CN, et al.: Epidemiology of bleeding in critically ill children. J Pediatr 184:114–119, 2017 - PubMed
-
- Pinto MG, Shabanova V, Li S, et al.: Epidemiology of clinically relevant bleeding in critically ill adolescents. Pediatr Crit Care Med 20:907–913, 2019 - PubMed
-
- Mitchell LG, Goldenberg NA, Male C, et al.: Definition of clinical efficacy and safety outcomes for clinical trials in deep venous thrombosis and pulmonary embolism in children. J Thromb Haemost 9:1856–1858, 2011 - PubMed
-
- Karam O, Nellis ME, Zantek ND, et al.: Criteria for clinically relevant bleeding in critically ill children: An international survey. Pediatr Crit Care Med 20:e137–e144, 2019 - PubMed
-
- Anderson MP, Cooper MT Jr.: The use of Bayesian analysis techniques in pediatric research. J Pediatr 205:295–297, 2019 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

