Efficacy comparison of commercial porcine circovirus type 2 (PCV2) and Mycoplasma hyopneumoniae monovalent and bivalent vaccines against a dual challenge
- PMID: 33012976
- PMCID: PMC7491006
Efficacy comparison of commercial porcine circovirus type 2 (PCV2) and Mycoplasma hyopneumoniae monovalent and bivalent vaccines against a dual challenge
Erratum in
-
Errata.Can J Vet Res. 2021 Jan;85(1):67. Can J Vet Res. 2021. PMID: 33390655 Free PMC article.
Abstract
The objective of this study was to compare the efficacy of commercially available porcine circovirus type 2 (PCV2) and Mycoplasma hyopneumoniae vaccines. A total of 80 pigs was randomly divided into 6 treatment groups; 4 of the groups each received a different vaccine as well as a dual challenge. The remaining 2 groups were used as controls, 1 of which also received a dual challenge. Two of the 4 groups of pigs were administered 2 monovalent vaccines (designated as either monovalent vaccine A or B) of PCV2 at 7 days old and of M. hyopneumoniae at 21 days old. The remaining 2 vaccinated groups of pigs received a bivalent vaccine (designated as either bivalent vaccine A or B) of PCV2 and M. hyopneumoniae at 21 days old. All 4 vaccinated groups were challenged with M. hyopneumoniae at 42 days old [-14 d post-challenge (dpc)], followed by a PCV2d challenge at 56 days old (0 dpc). All 4 vaccinated/challenged groups displayed a reduction in clinical signs, PCV2d viremia, nasal shedding of M. hyopneumoniae, and lung lesions compared with pigs in the unvaccinated and challenged groups. Vaccination and challenge improved growth performance and increased the immunologic responses (M. hyopneumoniae- and PCV2-specific antibodies and interferon-γ-secreting cells) when compared to pigs in the unvaccinated/challenged groups. Pigs in groups vaccinated with either a monovalent or bivalent vaccine A treatment and challenge produced a larger amount of M. hyopneumoniae- and PCV2d-specific interferon-γ-secreting cells within the pigs and simultaneously reduced the nasal shedding of M. hyopneumoniae and PCV2d viremia compared with groups vaccinated with either a monovalent or bivalent vaccine B treatment and challenge. Both the bivalent vaccines and the respective monovalent vaccines were efficacious against a dual challenge of M. hyopneumoniae and PCV2d.
L’objectif de la présente étude était de comparer l’efficacité de vaccins commercialement disponibles contre le circovirus porcin de type 2 (PCV2) et Mycoplasma hyopneumoniae. Un total de 80 porcs ont été divisés de manière aléatoire en six groupes de traitement; quatre des groupes ont chacun reçu un vaccin différent ainsi qu’une infection défi double. Les deux groupes restants ont servi de témoin, un des deux recevant l’infection défi double. Deux des quatre groupes de porcs ont reçu deux vaccins monovalents (désigné comme étant vaccin monovalent A ou B) de PCV2 à 7 jours d’âge et de M. hyopneumoniae à 21 jours d’âge. Les deux autres groupes de porcs vaccinés ont reçu un vaccin bivalent (désigné comme étant vaccin bivalent A ou B) de PCV2 et de M. hyopneumoniae à 21 jours d’âge. Les quatre groupes vaccinés furent challengés avec M. hyopneumoniae à 42 jours d’âge [−14 j post-défi (dpc)], suivi d’une infection défi avec PCV2 à 56 j d’âge (0 dpc). Les quatre groupes vaccinés/infectés ont montré une réduction des signes cliniques, de la virémie à PCV2d, de l’excrétion nasale de M. hyopneumoniae et de lésions pulmonaires comparativement aux porcs dans les groupes non-vaccinés et infectés. La vaccination et l’infection défi ont amélioré les performances de croissance et augmenté les réponses immunologiques (anticorps spécifiques contre M. hyopneumoniae et PCV2d et les cellules secrétant l’interféron-γ) lorsque comparé aux porcs dans les groupes non-vaccinés/infectés. Les porcs dans les groupes vaccinés avec soit le vaccin A monovalent ou bivalent et infectés ont produit de plus grandes quantités de cellules secrétant de l’interféron-γ spécifique à M. hyopneumoniae et PCV2d chez les porcs et ont simultanément réduit l’excrétion nasale de M. hyopneumoniae et la virémie de PCV2d comparativement aux groupes vaccinés avec le vaccin monovalent ou bivalent B et infectés. Autant les vaccins bivalents que les vaccins monovalents respectifs étaient efficaces à une infection défi double par M. hyopneumoniae et PCV2d.(Traduit par Docteur Serge Messier).
Copyright and/or publishing rights held by the Canadian Veterinary Medical Association.
Figures
 ); VacA-PM/Ch (
); VacA-PM/Ch (
 ); VacB-M+P/Ch (
); VacB-M+P/Ch (
 ); VacB-PM/Ch (
); VacB-PM/Ch (
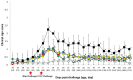
 ); UnVac/Ch (■); and UnVac/UnCh (△). Different letters within a sampling point mean statistically significant differences (P < 0.05).
); UnVac/Ch (■); and UnVac/UnCh (△). Different letters within a sampling point mean statistically significant differences (P < 0.05).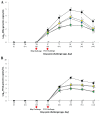
 ); VacA-PM/Ch (
); VacA-PM/Ch (
 ); VacB-M+P/Ch (
); VacB-M+P/Ch (
 ); VacB-PM/Ch (
); VacB-PM/Ch (
 ); UnVac/Ch (■); and UnVac/UnCh (△). Different letters within a sampling point mean statistically significant differences (P < 0.05).
); UnVac/Ch (■); and UnVac/UnCh (△). Different letters within a sampling point mean statistically significant differences (P < 0.05).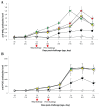
 ); VacA-PM/Ch (
); VacA-PM/Ch (
 ); VacB-M+P/Ch (
); VacB-M+P/Ch (
 ); VacB-PM/Ch (
); VacB-PM/Ch (
 ); UnVac/Ch (■); and UnVac/UnCh (△). Different letters within a sampling point mean statistically significant differences (P < 0.05).
); UnVac/Ch (■); and UnVac/UnCh (△). Different letters within a sampling point mean statistically significant differences (P < 0.05).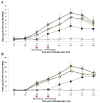
 ); VacA-PM/Ch (
); VacA-PM/Ch (
 ); VacB-M+P/Ch (
); VacB-M+P/Ch (
 ); VacB-PM/Ch (
); VacB-PM/Ch (
 ); UnVac/Ch (■); and UnVac/UnCh (△). Different letters within a sampling point mean statistically significant differences (P < 0.05).
); UnVac/Ch (■); and UnVac/UnCh (△). Different letters within a sampling point mean statistically significant differences (P < 0.05).Similar articles
-
Efficacy of a new bivalent vaccine of porcine circovirus type 2 and Mycoplasma hyopneumoniae (Fostera™ PCV MH) under experimental conditions.Vaccine. 2016 Jan 4;34(2):270-275. doi: 10.1016/j.vaccine.2015.11.034. Epub 2015 Nov 25. Vaccine. 2016. PMID: 26626212
-
Evaluation of the efficacy of a trivalent vaccine mixture against a triple challenge with Mycoplasma hyopneumoniae, PCV2, and PRRSV and the efficacy comparison of the respective monovalent vaccines against a single challenge.BMC Vet Res. 2019 Oct 16;15(1):342. doi: 10.1186/s12917-019-2091-6. BMC Vet Res. 2019. PMID: 31619295 Free PMC article.
-
Experimental efficacy of a trivalent vaccine containing porcine circovirus types 2a/b (PCV2a/b) and Mycoplasma hyopneumoniae against PCV2d and M. hyopneumoniae challenges.Vet Microbiol. 2021 Jul;258:109100. doi: 10.1016/j.vetmic.2021.109100. Epub 2021 May 4. Vet Microbiol. 2021. PMID: 33984792
-
Porcine Circovirus Type 2 (PCV2) Vaccines in the Context of Current Molecular Epidemiology.Viruses. 2017 May 6;9(5):99. doi: 10.3390/v9050099. Viruses. 2017. PMID: 28481275 Free PMC article. Review.
-
Porcine Circovirus Type 2 Vaccines: Commercial Application and Research Advances.Viruses. 2022 Sep 10;14(9):2005. doi: 10.3390/v14092005. Viruses. 2022. PMID: 36146809 Free PMC article. Review.
Cited by
-
Efficacy of Two Commercial Ready-To-Use PCV2 and Mycoplasma hyopneumoniae Vaccines under Field Conditions.Animals (Basel). 2021 May 26;11(6):1553. doi: 10.3390/ani11061553. Animals (Basel). 2021. PMID: 34073385 Free PMC article.
References
-
- Chae C. A review of porcine circovirus 2-associated syndromes and diseases. Vet J. 2005;169:326–336. - PubMed
-
- Davies B, Wang X, Dvorak CMT, Marthaler D, Murtaugh MP. Diagnostic phylogenetics reveals a new porcine circovirus 2 cluster. Virus Res. 2016;217:32–37. - PubMed
-
- Seo HW, Park C, Kang I, et al. Genetic and antigenic characterization of a newly emerging porcine circovirus type 2b mutant first isolated in cases of vaccine failure in Korea. Arch Virol. 2014;159:3107–3111. - PubMed
-
- Thacker EL, Minion C. Mycoplasmosis. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, editors. Diseases of Swine. 10th ed. Ames, Iowa: Wiley-Blackwell; 2012. pp. 779–797.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
