Potential Pathways for Circadian Dysfunction and Sundowning-Related Behavioral Aggression in Alzheimer's Disease and Related Dementias
- PMID: 33013301
- PMCID: PMC7494756
- DOI: 10.3389/fnins.2020.00910
Potential Pathways for Circadian Dysfunction and Sundowning-Related Behavioral Aggression in Alzheimer's Disease and Related Dementias
Abstract
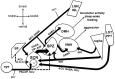
Patients with Alzheimer's disease (AD) and related dementias are commonly reported to exhibit aggressive behavior and other emotional behavioral disturbances, which create a tremendous caretaker burden. There has been an abundance of work highlighting the importance of circadian function on mood and emotional behavioral regulation, and recent evidence demonstrates that a specific hypothalamic pathway links the circadian system to neurons that modulate aggressive behavior, regulating the propensity for aggression across the day. Such shared circuitry may have important ramifications for clarifying the complex interactions underlying "sundowning syndrome," a poorly understood (and even controversial) clinical phenomenon in AD and dementia patients that is characterized by agitation, aggression, and delirium during the late afternoon and early evening hours. The goal of this review is to highlight the potential output and input pathways of the circadian system that may underlie circadian dysfunction and behavioral aggression associated with sundowning syndrome, and to discuss possible ways these pathways might inform specific interventions for treatment. Moreover, the apparent bidirectional relationship between chronic disruptions of circadian and sleep-wake regulation and the pathology and symptoms of AD suggest that understanding the role of these circuits in such neurobehavioral pathologies could lead to better diagnostic or even preventive measures.
Keywords: Alzheimer’s disease; aggression; agitation; circadian; dementia; sundowning.
Copyright © 2020 Todd.
Figures
References
-
- Aging N. I. O. (2017). Tips for Coping with Sundowning [Online]. Available: https://www.nia.nih.gov/health/tips-coping-sundowning (accessed May 1, 2020).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

