The Structure, Function, and Physiology of the Fetal and Adult Acetylcholine Receptor in Muscle
- PMID: 33013323
- PMCID: PMC7506097
- DOI: 10.3389/fnmol.2020.581097
The Structure, Function, and Physiology of the Fetal and Adult Acetylcholine Receptor in Muscle
Abstract
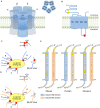
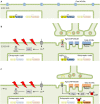
The neuromuscular junction (NMJ) is a highly developed synapse linking motor neuron activity with muscle contraction. A complex of molecular cascades together with the specialized NMJ architecture ensures that each action potential arriving at the motor nerve terminal is translated into an action potential in the muscle fiber. The muscle-type nicotinic acetylcholine receptor (AChR) is a key molecular component located at the postsynaptic muscle membrane responsible for the generation of the endplate potential (EPP), which usually exceeds the threshold potential necessary to activate voltage-gated sodium channels and triggers a muscle action potential. Two AChR isoforms are found in mammalian muscle. The fetal isoform is present in prenatal stages and is involved in the development of the neuromuscular system whereas the adult isoform prevails thereafter, except after denervation when the fetal form is re-expressed throughout the muscle. This review will summarize the structural and functional differences between the two isoforms and outline congenital and autoimmune myasthenic syndromes that involve the isoform specific AChR subunits.
Keywords: adult acetylcholine receptor; fetal acetylcholine receptor; ion channel; muscle development; myasthenia; neuromuscular junction.
Copyright © 2020 Cetin, Beeson, Vincent and Webster.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

