Homeostatic Roles of the Proteostasis Network in Dendrites
- PMID: 33013325
- PMCID: PMC7461941
- DOI: 10.3389/fncel.2020.00264
Homeostatic Roles of the Proteostasis Network in Dendrites
Abstract
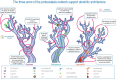
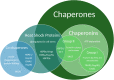
Cellular protein homeostasis, or proteostasis, is indispensable to the survival and function of all cells. Distinct from other cell types, neurons are long-lived, exhibiting architecturally complex and diverse multipolar projection morphologies that can span great distances. These properties present unique demands on proteostatic machinery to dynamically regulate the neuronal proteome in both space and time. Proteostasis is regulated by a distributed network of cellular processes, the proteostasis network (PN), which ensures precise control of protein synthesis, native conformational folding and maintenance, and protein turnover and degradation, collectively safeguarding proteome integrity both under homeostatic conditions and in the contexts of cellular stress, aging, and disease. Dendrites are equipped with distributed cellular machinery for protein synthesis and turnover, including dendritically trafficked ribosomes, chaperones, and autophagosomes. The PN can be subdivided into an adaptive network of three major functional pathways that synergistically govern protein quality control through the action of (1) protein synthesis machinery; (2) maintenance mechanisms including molecular chaperones involved in protein folding; and (3) degradative pathways (e.g., Ubiquitin-Proteasome System (UPS), endolysosomal pathway, and autophagy. Perturbations in any of the three arms of proteostasis can have dramatic effects on neurons, especially on their dendrites, which require tightly controlled homeostasis for proper development and maintenance. Moreover, the critical importance of the PN as a cell surveillance system against protein dyshomeostasis has been highlighted by extensive work demonstrating that the aggregation and/or failure to clear aggregated proteins figures centrally in many neurological disorders. While these studies demonstrate the relevance of derangements in proteostasis to human neurological disease, here we mainly review recent literature on homeostatic developmental roles the PN machinery plays in the establishment, maintenance, and plasticity of stable and dynamic dendritic arbors. Beyond basic housekeeping functions, we consider roles of PN machinery in protein quality control mechanisms linked to dendritic plasticity (e.g., dendritic spine remodeling during LTP); cell-type specificity; dendritic morphogenesis; and dendritic pruning.
Keywords: autophagy; chaperone; dendrite; developmental homeostasis; neurological disease; proteostasis network; ribosome; ubiquitin-proteasome system (UPS).
Copyright © 2020 Lottes and Cox.
Figures
Similar articles
-
The disturbance of protein synthesis/degradation homeostasis is a common trait of age-related neurodegenerative disorders.Adv Protein Chem Struct Biol. 2022;132:49-87. doi: 10.1016/bs.apcsb.2022.05.008. Epub 2022 Jun 9. Adv Protein Chem Struct Biol. 2022. PMID: 36088079
-
Molecular chaperones and proteostasis regulation during redox imbalance.Redox Biol. 2014 Jan 30;2:323-32. doi: 10.1016/j.redox.2014.01.017. eCollection 2014. Redox Biol. 2014. PMID: 24563850 Free PMC article. Review.
-
Proteasome-mediated proteostasis: Novel medicinal and pharmacological strategies for diseases.Med Res Rev. 2018 Sep;38(6):1916-1973. doi: 10.1002/med.21502. Epub 2018 May 2. Med Res Rev. 2018. PMID: 29719055 Review.
-
Cleaning the molecular machinery of cells via proteostasis, proteolysis and endocytosis selectively, effectively, and precisely: intracellular self-defense and cellular perturbations.Mol Omics. 2021 Feb 1;17(1):11-28. doi: 10.1039/d0mo00085j. Epub 2020 Nov 2. Mol Omics. 2021. PMID: 33135707
-
Potential Influence of Cyclo(His-Pro) on Proteostasis: Impact on Neurodegenerative Diseases.Curr Protein Pept Sci. 2018;19(8):805-812. doi: 10.2174/1389203719666180430155112. Curr Protein Pept Sci. 2018. PMID: 29708066 Review.
Cited by
-
A Combined Proteomics and Metabolomics Profiling to Investigate the Genetic Heterogeneity of Autistic Children.Mol Neurobiol. 2022 Jun;59(6):3529-3545. doi: 10.1007/s12035-022-02801-x. Epub 2022 Mar 28. Mol Neurobiol. 2022. PMID: 35348996
-
Improper Proteostasis: Can It Serve as Biomarkers for Neurodegenerative Diseases?Mol Neurobiol. 2022 Jun;59(6):3382-3401. doi: 10.1007/s12035-022-02775-w. Epub 2022 Mar 19. Mol Neurobiol. 2022. PMID: 35305242 Review.
-
Synaptic dysfunction in ALS and FTD: anatomical and molecular changes provide insights into mechanisms of disease.Front Mol Neurosci. 2022 Oct 3;15:1000183. doi: 10.3389/fnmol.2022.1000183. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36263379 Free PMC article. Review.
-
PP2A phosphatase regulates cell-type specific cytoskeletal organization to drive dendrite diversity.Front Mol Neurosci. 2022 Nov 14;15:926567. doi: 10.3389/fnmol.2022.926567. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36452406 Free PMC article.
-
Ca2+ channels couple spiking to mitochondrial metabolism in substantia nigra dopaminergic neurons.Sci Adv. 2022 Sep 30;8(39):eabp8701. doi: 10.1126/sciadv.abp8701. Epub 2022 Sep 30. Sci Adv. 2022. PMID: 36179023 Free PMC article.
References
-
- Aguirre-Chen C., Stec N., Ramos O. M., Kim N., Kramer M., McCarthy S., et al. (2020). A Caenorhabditis elegans model for integrating the functions of neuropsychiatric risk genes identifies components required for normal dendritic morphology. G3 10 1617–1628. 10.1534/g3.119.400925 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

