Episodic Memories: How do the Hippocampus and the Entorhinal Ring Attractors Cooperate to Create Them?
- PMID: 33013334
- PMCID: PMC7511719
- DOI: 10.3389/fnsys.2020.559186
Episodic Memories: How do the Hippocampus and the Entorhinal Ring Attractors Cooperate to Create Them?
Abstract
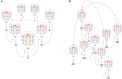
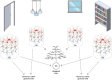
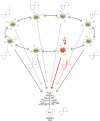
The brain is capable of registering a constellation of events, encountered only once, as an episodic memory that can last for a lifetime. As evidenced by the clinical case of the patient HM, memories preserving their episodic nature still depend on the hippocampal formation, several years after being created, while semantic memories are thought to reside in neocortical areas. The neurobiological substrate of one-time learning and life-long storing in the brain, that must exist at the cellular and circuit level, is still undiscovered. The breakthrough is delayed by the fact that studies jointly investigating the rodent hippocampus and entorhinal cortex are mostly targeted at understanding the spatial aspect of learning. Here, we present the concept of an entorhinal cortical module, termed EPISODE module, that could explain how the representations of different elements constituting episodic memories can be linked together at the stage of encoding. The new model that we propose here reconciles the structural and functional observations made in the entorhinal cortex and explains how the downstream hippocampal processing organizes the representations into meaningful sequences.
Keywords: entorhinal cortex; episodic memory; grid cells; hippocampus; phase precession; plasticity; theta phase skipping; theta sequences.
Copyright © 2020 Kovács.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

