Bronchial Vascular Remodeling Is Attenuated by Anti-IL-17 in Asthmatic Responses Exacerbated by LPS
- PMID: 33013361
- PMCID: PMC7500412
- DOI: 10.3389/fphar.2020.01269
Bronchial Vascular Remodeling Is Attenuated by Anti-IL-17 in Asthmatic Responses Exacerbated by LPS
Abstract
Introduction: Although the major alterations associated with asthma are related to the airways, there is also evidence of the importance of peribronchial vascular inflammation and remodeling in its pathophysiology.
Objectives: To determine the effects of anti-IL-17 therapy on peribronchial vessels of an asthma model exacerbated by lipopolysaccharide.
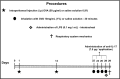
Methods: We evaluated several factors, including lung function, inflammation, oxidative stress, vascular remodeling, and signaling pathways present in the peribronchial vessels of 66 male BALB/c mice exposed to ovalbumin and treated (or not) treated with anti-IL-17. Twenty-four hours before the end of the experimental protocol, groups of sensitized animals (OVA-LPS and OVA-LPS anti-IL-17) also received LPS.
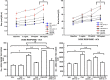
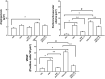
Results: The OVA-LPS-anti-IL-17 group presented a decrease in several factors [airway resistance and elastance, bronchoalveolar lavage fluid (BALF) cell counts, inflammatory response, eosinophils, TSLP, IL-33, TARC, TNF-α, CD4+, CD8+, IL-4, IL-6, IL-10, IL-17, and VEGF positive cells/104μm2, peribronchovascular edema, and angiogenesis], including remodeling (MMP-9, MMP-12, TIMP-1 and TGF-β positive cells and volume fraction of collagen fibers I, collagen fibers III, collagen fibers V, decorin, lumican, actin, biglycan, fibronectin, and integrin), oxidative stress (iNOS positive cells and volume fraction of PGF2α), and signaling pathways (FoxP3), as well as dendritic cells, NF-kB, ROCK-1, ROCK-2, STAT-1, and phosphor-STAT1-positive cells compared to OVA-LPS (p < 0.05).
Conclusions: In this model of LPS-induced asthma exacerbation, IL-17 inhibition represents a promising therapeutic strategy, indicating the potential of bronchial vascular control of Th2 and Th17 responses and the activation of the remodeling and oxidative stress pathways, associated with the control of signaling pathways.
Keywords: LPS (lipopolysaccharide); anti-IL-17; asthma; vascular inflammation; vascular remodeling.
Copyright © 2020 Camargo, Santos, Andrade, Fukuzaki, dos Santos Lopes, de Arruda Martins, Prado, Leick, Righetti and Tibério.
Figures








References
-
- Angeli P., Prado C. M., Xisto D. G., Silva P. L., Passaro C. P., Nakazato H. D., et al. (2008). Effects of chronic L-NAME treatment lung tissue mechanics, eosinophilic and extracellular matrix responses induced by chronic pulmonary inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 294 (6), L1197–L1205. 10.1152/ajplung.00199.2007 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

