Unraveling the Pharmacological Potential of Lichen Extracts in the Context of Cancer and Inflammation With a Broad Screening Approach
- PMID: 33013369
- PMCID: PMC7509413
- DOI: 10.3389/fphar.2020.01322
Unraveling the Pharmacological Potential of Lichen Extracts in the Context of Cancer and Inflammation With a Broad Screening Approach
Abstract
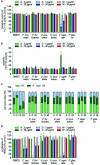
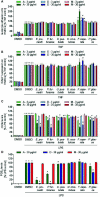
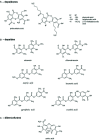
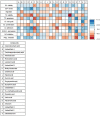
Lichen-forming fungi are symbiotic organisms that synthesize unique natural products with potential for new drug leads. Here, we explored the pharmacological activity of six lichen extracts (Evernia prunastri, Pseudevernia furfuracea, Umbilicaria pustulata, Umbilicaria crustulosa, Flavoparmelia caperata, Platismatia glauca) in the context of cancer and inflammation using a comprehensive set of 11 functional and biochemical in vitro screening assays. We assayed intracellular Ca2+ levels and cell migration. For cancer, we measured tumor cell proliferation, cell cycle distribution and apoptosis, as well as the angiogenesis-associated proliferation of endothelial cells (ECs). Targeting inflammation, we assayed leukocyte adhesion onto ECs, EC adhesion molecule expression, as well as nitric oxide production and prostaglandin (PG)E2 synthesis in leukocytes. Remarkably, none of the lichen extracts showed any detrimental influence on the viability of ECs. We showed for the first time that extracts of F. caperata induce Ca2+ signaling. Furthermore, extracts from E. prunastri, P. furfuracea, F. caperata, and P. glauca reduced cell migration. Interestingly, F. caperata extracts strongly decreased tumor cell survival. The proliferation of ECs was significantly reduced by E. prunastri, P. furfuracea, and F. caperata extracts. The extracts did not inhibit the activity of inflammatory processes in ECs. However, the pro-inflammatory activation of leukocytes was inhibited by extracts from E. prunastri, P. furfuracea, F. caperata, and P. glauca. After revealing the potential biological activities of lichen extracts by an array of screening tests, a correlation analysis was performed to evaluate particular roles of abundant lichen secondary metabolites, such as atranorin, physodic acid, and protocetraric acid as well as usnic acid in various combinations. Overall, some of the lichen extracts tested in this study exhibit significant pharmacological activity in the context of inflammation and/or cancer, indicating that the group lichen-forming fungi includes promising members for further testing.
Keywords: cancer; cytotoxicity; inflammation; lichen extracts; migration; screening.
Copyright © 2020 Ingelfinger, Henke, Roser, Ulshöfer, Calchera, Singh, Parnham, Geisslinger, Fürst, Schmitt and Schiffmann.
Figures


References
-
- Asplund J., Bokhorst S., Kardol P., Wardle D. A. (2015). Removal of secondary compounds increases invertebrate abundance in lichens. Fungal Ecol. 18, 18–25. 10.1016/j.funeco.2015.07.009 - DOI
-
- Bauer J., Waltenberger B., Noha S. M., Schuster D., Rollinger J. M., Boustie J., et al. (2012). Discovery of depsides and depsidones from lichen as potent inhibitors of microsomal prostaglandin E2 synthase-1 using pharmacophore models. ChemMedChem 7 (12), 2077–2081. 10.1002/cmdc.201200345 - DOI - PMC - PubMed
-
- Benatti M. N., Gernert M., Schmitt I. (2013). Parmotrema hydrium, a new species of Parmeliaceae in southeastern Brazil. Acta Botanica Brasilica 27 (4), 810–814. 10.1590/S0102-33062013000400021 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

