Aucubin Attenuates Liver Ischemia-Reperfusion Injury by Inhibiting the HMGB1/TLR-4/NF-κB Signaling Pathway, Oxidative Stress, and Apoptosis
- PMID: 33013386
- PMCID: PMC7506056
- DOI: 10.3389/fphar.2020.544124
Aucubin Attenuates Liver Ischemia-Reperfusion Injury by Inhibiting the HMGB1/TLR-4/NF-κB Signaling Pathway, Oxidative Stress, and Apoptosis
Abstract
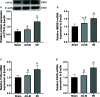
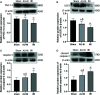
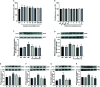
Liver ischemia-reperfusion injury (IRI) is a common clinical event with high morbidity in patients undergoing complex liver surgery or having abdominal trauma. Inflammatory and oxidative stress responses are the main contributing factors in liver IRI. The iridoid glucoside aucubin (AU) has good anti-inflammatory and antioxidative effects; however, there are no relevant reports on the protective effect of glucosides on hepatic IRI. The purpose of this study was to determine whether AU pretreatment could prevent liver IRI and to explore the mechanism. Sprague-Dawley rats were randomly divided into five groups. The sham operation and IRI control groups were given intraperitoneal injections of normal saline, while the AU low-dose (AU-L) group, AU medium-dose (AU-M) group, and AU high-dose (AU-H) group were given intraperitoneal injections of AU at doses of 1, 5, and 10 mg/kg/day, respectively. After 10 d, liver IRI (70% liver ischemia for 1 h, reperfusion for 6 h) was surgically established in all groups except the sham group. Our results confirmed that liver injury was significantly aggravated after hepatic ischemia-reperfusion. AU alleviated the increase of transaminase and pathological changes induced by ischemia-reperfusion and improved liver damage. AU could also ameliorate the inflammatory and oxidative stress responses induced by ischemia-reperfusion and reduced expression of high mobility group protein (HMG)B1, receptor for advanced glycation end-products (RAGE), tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and reactive oxygen species (ROS). Moreover, AU reduced ischemia-reperfusion-induced mitochondrial dysfunction and cells apoptosis, increased peroxisome proliferator-activated receptor γ coactivator (PGC)-1α and uncoupling (UCP)2 protein expression, and reduced caspase-3, cleaved caspase-3, and Cytochrome P450 proteins (CYP) expression. To determine expression levels of the Toll-like receptor (TLR)-4/nuclear factor-κB (NF-κB) pathway-related proteins in vitro and in vivo, we also measured TLR-4, myeloid differentiation factor88 (MyD88), NF-κB P65, p-P65, I-kappa-B-alpha (IκB-α), and p-IκB-α levels. The results showed that AU effectively inhibited activation of the TLR-4/NF-κB signaling pathway. In conclusion, we showed for the first time a hepatoprotective effect for AU in liver IRI, which acted by inhibiting the HMGB1/TLR-4/NF-κB signaling pathway, oxidative stress, and apoptosis. Pretreatment with AU may be a promising strategy for preventing liver IRI.
Keywords: apoptosis; aucubin; inflammation; ischemia-reperfusion; liver; oxidative stress.
Copyright © 2020 Zhang, Feng, Gao, Duan, Fan, Geng, Wu, Li, Liu and Peng.
Figures





References
-
- Bi J., Zhang J., Ren Y., Du Z., Li Q., Wang Y., et al. (2019). Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biol. 20, 296–306. 10.1016/j.redox.2018.10.019 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

