Effect of Triptolide on Temporal Expression of Cell Cycle Regulators During Cardiac Hypertrophy
- PMID: 33013405
- PMCID: PMC7498627
- DOI: 10.3389/fphar.2020.566938
Effect of Triptolide on Temporal Expression of Cell Cycle Regulators During Cardiac Hypertrophy
Abstract
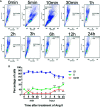
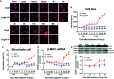
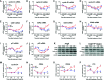
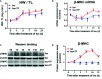
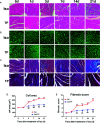
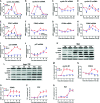
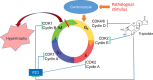
Adult mammalian cardiomyocytes may reenter the cell cycle and cause cardiac hypertrophy. Triptolide (TP) can regulate the expressions of various cell cycle regulators in cancer cells. However, its effects on cell cycle regulators during myocardial hypertrophy and mechanism are unclear. This study was designed to explore the profile of cell cycle of cardiomyocytes and the temporal expression of their regulators during cardiac hypertrophy, as well as the effects of TP. The hypertrophy models employed were neonatal rat ventricular myocytes (NRVMs) stimulated with angiotensin II (Ang II) for scheduled times (from 5 min to 48 h) in vitro and mice treated with isoprenaline (Iso) for from 1 to 21 days, respectively. TP was used in vitro at 1 μg/L and in vivo at 10 μg/kg. NRVMs were analyzed using flow cytometry to detect the cell cycle, and the expression levels of mRNA and protein of various cell cycle regulators were determined using real-time PCR and Western blot. It was found NRVM numbers in phases S and G2 increased, while that in the G1 phase decreased significantly after Ang II stimulation. The mRNA expression levels of p21 and p27 increased soon after stimulation, and thereafter, mRNA expression levels of all cell cycle factors showed a decreasing trend and reached their lowest levels in 1-3 h, except for cyclin-dependent kinase 1 (CDK1) and CDK4 mRNA. The mRNA expression levels of CDK1, p21, and p27 increased markedly after stimulation with Ang II for 24-48 h. In myocardium tissue, CDK and cyclin expression levels peaked in 3-7 days, followed by a decreasing trend, while those of p21 and p27 mRNA remained at a high level on day 21. Expression levels of all protein were consistent with the results of mRNA in NRVMs or mice. The influence of Ang II or Iso on protein expression was more obvious than that on mRNA. TP treatment effectively prevented the imbalance in the expression of cell cycle regulators in the hypertrophy model group. In Conclusion, an imbalance in the expression of cell cycle regulators occurs during cardiac hypertrophy, and triptolide corrects these abnormal expression levels and attenuates cardiac hypertrophy.
Keywords: cardiac hypertrophy; cardiomyocyte; cell cycle; cyclin; cyclin-dependent kinase; triptolide.
Copyright © 2020 Li, Pan, Ding, Tong, Chen, Liu and Zhang.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

