Changes of Ocular Dimensions as a Marker of Disease Progression in a Murine Model of Pigmentary Glaucoma
- PMID: 33013417
- PMCID: PMC7500411
- DOI: 10.3389/fphar.2020.573238
Changes of Ocular Dimensions as a Marker of Disease Progression in a Murine Model of Pigmentary Glaucoma
Abstract
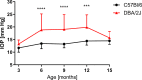
Purpose: The elevation of intraocular pressure (IOP), a major risk factor in glaucoma, is an important parameter tracked in experimental models of this disease. However, IOP measurement in laboratory rodents is challenging and may not correlate with some key pathological events that occur in the development of glaucoma. The aims of this study were to quantify changes in ocular morphology in DBA/2J mice that develop spontaneous, age-dependent, pigmentary glaucoma and to check the possible correlation of these parameters with IOP.
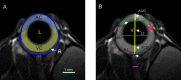
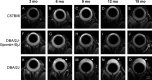
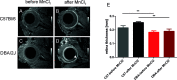
Method: Eye morphology was evaluated with MRI in DBA/2J, DBA/2J-Gpnmb+/SjJ, and C57BL/6J female mice ages 3, 6, 9, 12, and 15 months. The animals were anesthetized with isoflurane. A planar receive-only surface coil (inner diameter = 10 mm) was placed over each animal's left eye and the image was acquired with a 7T small animal-dedicated magnetic resonance tomograph and T2-weighted TurboRARE sequence. Ocular dimensions were manually quantitated using OsiriX software. IOP was measured with rebound tonometry.
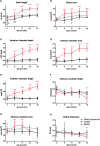
Results: In the control animals, no age-related changes in the ocular morphology were noted. Since 6 months of age, the anterior chamber deepening and elongation of the eyeballs of DBA/2J mice was detectable. We found a significant, positive correlation between IOP and axial length, anterior chamber area, or anterior chamber width in C57BL/6J mice but not in DBA/2J mice. However, after excluding the measurements performed in the oldest DBA/2J mice (i.e. analyzing only the animals ages 3 to 12 months), we demonstrated a significant positive correlation between IOP and anterior chamber width.
Conclusion: High-resolution magnetic resonance imaging of the eye area in mice enables reproducible and consistent measures of key dimensions of the eyeball. We observed age-dependent alterations in the eye morphology of DBA/2J mice that mostly affected the anterior chamber. We also demonstrated a correlation between some of the ocular dimensions and the IOP of C57Bl/6J mice and DBA/2J mice with moderately advanced glaucomatous pathology.
Keywords: DBA/2J; MRI; glaucoma; intraocular pressure; ocular biomechanics; ocular dimensions.
Copyright © 2020 Fiedorowicz, Wełniak-Kamińska, Świątkiewicz, Orzeł, Chorągiewicz, Toro, Rejdak, Bogorodzki and Grieb.
Figures

Similar articles
-
The Contribution of Anterior Segment Abnormalities to Changes in Intraocular Pressure in the DBA/2J Mouse Model of Glaucoma: DBA/2J-Gpnmb +/SjJ Mice as Critical Controls.Front Neurosci. 2022 Feb 3;15:801184. doi: 10.3389/fnins.2021.801184. eCollection 2021. Front Neurosci. 2022. PMID: 35185449 Free PMC article.
-
Age-related changes in eye morphology and aqueous humor dynamics in DBA/2J mice using contrast-enhanced ocular MRI.Magn Reson Imaging. 2019 Jun;59:10-16. doi: 10.1016/j.mri.2019.01.016. Epub 2019 Jan 17. Magn Reson Imaging. 2019. PMID: 30660703
-
Dependency of intraocular pressure elevation and glaucomatous changes in DBA/2J and DBA/2J-Rj mice.Invest Ophthalmol Vis Sci. 2008 Feb;49(2):613-21. doi: 10.1167/iovs.07-0745. Invest Ophthalmol Vis Sci. 2008. PMID: 18235006
-
Anterograde Transport in Axons of the Retinal Ganglion Cells and its Relationship to the Intraocular Pressure during Aging in Mice with Hereditary Pigmentary Glaucoma.Curr Eye Res. 2018 Apr;43(4):539-546. doi: 10.1080/02713683.2017.1416147. Epub 2017 Dec 28. Curr Eye Res. 2018. PMID: 29283693
-
Cochlin and glaucoma: a mini-review.Vis Neurosci. 2005 Sep-Oct;22(5):605-13. doi: 10.1017/S0952523805225099. Vis Neurosci. 2005. PMID: 16332271 Free PMC article. Review.
Cited by
-
Morphometric Analysis of the Eye by Magnetic Resonance Imaging in MGST2-Gene-Deficient Mice.Biomedicines. 2024 Feb 5;12(2):370. doi: 10.3390/biomedicines12020370. Biomedicines. 2024. PMID: 38397974 Free PMC article.
-
Tryptophan Pathway Abnormalities in a Murine Model of Hereditary Glaucoma.Int J Mol Sci. 2021 Jan 21;22(3):1039. doi: 10.3390/ijms22031039. Int J Mol Sci. 2021. PMID: 33494373 Free PMC article.
-
The Effect of Ocular Perfusion Pressure on Retinal Thickness in Young People with Presumed Systemic Hypotension.Vision (Basel). 2021 Jul 14;5(3):36. doi: 10.3390/vision5030036. Vision (Basel). 2021. PMID: 34287377 Free PMC article.
-
Influence of Trace Elements on Neurodegenerative Diseases of The Eye-The Glaucoma Model.Int J Mol Sci. 2021 Apr 21;22(9):4323. doi: 10.3390/ijms22094323. Int J Mol Sci. 2021. PMID: 33919241 Free PMC article. Review.
-
Modeling Retinal Ganglion Cell Dysfunction in Optic Neuropathies.Cells. 2021 Jun 5;10(6):1398. doi: 10.3390/cells10061398. Cells. 2021. PMID: 34198840 Free PMC article. Review.
References
-
- Anon (2020). DBA/2J-Gpnmb+/SjJ Stock No: 007048 (Jackson Laboratory; ). Available at: https://www.jax.org/strain/007048 (Accessed 07-05-2020).
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

