Stromal Cells Promote Neovascular Invasion Across Tissue Interfaces
- PMID: 33013445
- PMCID: PMC7461918
- DOI: 10.3389/fphys.2020.01026
Stromal Cells Promote Neovascular Invasion Across Tissue Interfaces
Abstract
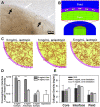
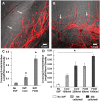
Vascular connectivity between adjacent vessel beds within and between tissue compartments is essential to any successful neovascularization process. To establish new connections, growing neovessels must locate other vascular elements during angiogenesis, often crossing matrix and other tissue-associated boundaries and interfaces. How growing neovessels traverse any tissue interface, whether part of the native tissue structure or secondary to a regenerative procedure (e.g., an implant), is not known. In this study, we developed an experimental model of angiogenesis wherein growing neovessels must interact with a 3D interstitial collagen matrix interface that separates two distinct tissue compartments. Using this model, we determined that matrix interfaces act as a barrier to neovessel growth, deflecting growing neovessels parallel to the interface. Computational modeling of the neovessel/matrix biomechanical interactions at the interface demonstrated that differences in collagen fibril density near and at the interface are the likely mechanism of deflection, while fibril alignment guides deflected neovessels along the interface. Interestingly, stromal cells facilitated neovessel interface crossing during angiogenesis via a vascular endothelial growth factor (VEGF)-A dependent process. However, ubiquitous addition of VEGF-A in the absence of stromal cells did not promote interface invasion. Therefore, our findings demonstrate that vascularization of a tissue via angiogenesis involves stromal cells providing positional cues to the growing neovasculature and provides insight into how a microvasculature is organized within a tissue.
Keywords: VEGF; neovessel invasion; stromal cells; tissue interface; vascular biology.
Copyright © 2020 Strobel, LaBelle, Krishnan, Dale, Rauff, Poulson, Bader, Beare, Aliaj, Weiss and Hoying.
Figures






References
-
- Breier G. (2000). Angiogenesis in embryonic development–a review. Placenta 21(Suppl. A), S11–S15. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

